Syringic Acid Improves Cholestatic Liver Disease by Regulating Bile Acid Metabolism and Intestinal Barrier
-
摘要:
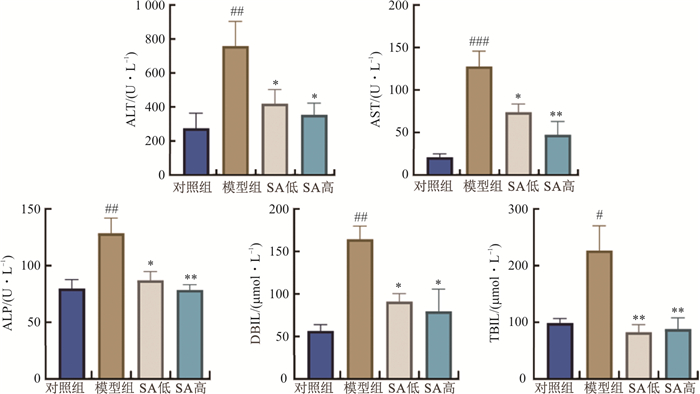
目的 基于胆汁酸代谢和肠道屏障探讨丁香酸对胆汁淤积小鼠的调控作用。 方法 20只小鼠随机分为对照组、模型组和丁香酸低、高剂量(70、140 mg·kg-1)组。连续7 d灌胃给予相应药物, 于第5天给药2 h后腹腔注射α-萘异硫氰酸酯(ANIT)诱导肝内胆汁淤积小鼠模型。末次给药后, 记录小鼠体质量及肝质量变化; 检测小鼠血清中肝功能指标、组织病理学变化, qPCR验证小鼠结肠组织中紧密连接蛋白闭锁小带蛋白-1(ZO-1)、闭合蛋白(Occludin)、紧密连接蛋白-5(Claudin-5) mRNA的表达, 采用非靶向代谢组学分析血清中代谢产物的变化, 检测肝脏和粪便中总胆汁酸变化。 结果 丁香酸可以显著降低模型组小鼠血清中丙氨酸氨基转移酶(ALT)、天冬氨酸氨基转移酶(AST)、碱性磷酸酶(ALP)活性和总胆红素(TBIL)、直接胆红素(DBIL)水平(P<0.05,P<0.01), 减轻肝脏损伤和坏死; 降低模型组小鼠结肠的淋巴细胞浸润, 恢复隐窝形态。丁香酸高剂量组能显著升高结肠中ZO-1、Occludin、Claudin-5的mRNA表达水平(P<0.05);显著上调11个代谢物, 下调29个代谢物, 代谢产物主要涉及次生代谢物的生物合成、次级胆汁酸生物合成和胆汁分泌通路。丁香酸降低肝脏中总胆汁酸含量及增加粪便总胆汁酸的排出(P<0.05,P<0.01)。 结论 丁香酸可以显著改善胆汁淤积小鼠肠道屏障的受损, 并且促进胆汁酸的代谢, 这可能是丁香酸改善胆汁淤积的关键调控环节。 Abstract:OBJECTIVE To explore the regulatory effect of syringic acid in cholestatic mice based on bile acid metabolism and intestinal barrier. METHODS Twenty mice were randomly divided into control group, model group and low and high dose of syringic acid (70, 140 mg·kg-1) groups. Intrahepatic cholestasis was induced by intraperitoneal injection of α-naphthalene isothiocyanate after 2 h of administration on the fifth day. After the last dose, the changes of body weight and liver mass of mice were recorded. Liver function indexes in serum and histopathology were detected, qPCR verified the expression of tight junction proteins Zonula Occludens Protein 1 (ZO-1), Occludin and Claudin-5 in mouse colon tissues, the changes of metabolites in serum were analyzed by using nontargeted metabolomics, and the changes of total bile acids in liver and feces were detected. RESULTS Syringic acid could significantly reduce the serum alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) activity, total bilirubin (TBIL) and direct bilirubin (DBIL) levels (P < 0.05, P < 0.01) in the model group mice, and reduce liver damage and necrosis. Syringic acid reduced lymphocyte infiltration in the colon of mice in the model group and restored crypt morphology, while the high-dose syringic acid group significantly increased the mRNA expression levels of ZO-1, Occludin, and Claudin-5 in the colon (P < 0.05). The high-dose intervention of syringic acid significantly upregulated 11 metabolites and 29 metabolites, and the metabolites mainly involved the biosynthesis of secondary metabolites, secondary bile acid biosynthesis and bile secretion pathways. Syringic acid reduced the content of total bile acids in the liver and increased the excretion of total bile acids in feces (P < 0.05, P < 0.01). CONCLUSION Syringic acid can significantly improve the phenotype of cholestasis in cholestatic mice, improve the damage of intestinal barrier, and promote the metabolism of bile acids in cholestatic mice, which may be the key pathway for syringic acid to improve cholestasis. -
Key words:
- syringic acid /
- cholestasis /
- liver damage /
- intestinal barrier /
- metabolism of bile acids
-
表 1 引物序列
Table 1. Primer sequence
引物 正向(5’→3’) 反向(3’→5’) Gapdh AGGTCGGTGTGAACGGATTTG TGTAGACCATGTAGTTGAGGTCA ZO-1 AACCCGAAACTGATGCTGTGGATAG CGCCCTTGGAATGTATGTGGAGAG Occludin TGGCTATGGAGGCGGCTATGG GCGATGAAGCAGAAGG Claudin5 CTGCCTTCCTGGACCACAACATC CACCACGCACGACATCCACAG 表 2 丁香酸对ANIT小鼠肝脏指数的影响(x±s, n=5)
Table 2. Effect of syringic acid on liver indexin ANIT mice (x±s, n=5)
组别 体质量/g 肝脏质量/g 肝脏指数/% 对照组 22.92±0.77 1.16±0.07 5.08±0.22 模型组 20.82±0.40 1.32±0.05 6.36±0.31## SA低剂量组 22.30±0.53 1.11±0.04 4.96±0.15** SA高剂量组 23.22±0.47 1.17±0.06 5.02±0.21** 注: 与对照组比较, ##P<0.01;与模型组比较, * *P<0.01。 表 3 SA高剂量组调控的血清代谢物
Table 3. Serum metabolites regulated by the SA high dose group
编号 代谢物 TR/min Calc. MW 碎片峰 ESI Log2 FC 1 9Z-Octadecen-12-ynoicacid 7.53 278.224 7 219.056 0, 201.046 7, 149.023 8, 95.086 1 ESI+ 1.13 2 2-Oxindole 1.98 133.053 1 134.060 4, 106.065 8, 79.055 1 ESI+ -1.18 3 3'-hydroxyacetophenone 4.46 136.052 7 122.036 6, 94.042 0, 79.055 0 ESI+ 5.17 4 4-(2-Aminophenyl)-2_4-dioxobutanoate 1.64 207.053 6 162.055 3, 134.096 9, 84.960 7 ESI+ -3.63 5 Arachidoyl Ethanolamide 9.10 355.345 2 338.341 7, 106.086 7, 88.976 5 ESI+ -4.23 6 Corticosterone 4.15 346.214 5 329.2120, 123.081 1, 97.065 5 ESI+ 2.09 7 Hippuric acid 1.87 179.058 6 105.034 1, 95.0499 4, 77.039 4 ESI+ -1.61 8 Nonivamide 4.46 293.199 2 229.958 2, 137.060 0 ESI+ 5.78 9 Palmitoylcarnitine 9.50 399.335 0 341.268 4, 144.102 1, 95.086 5, 85.029 1, 57.070 6 ESI+ -7.05 10 Muroctasin 21.43 886.559 4 675.478 1, 303.233 7, 283.264 3, 241.012 0, 152.995 3, 78.958 0 ESI- 3.12 11 (3S, 5Z, 7E)-26, 26, 26, 27, 27, 27-Hexafluoro-9, 10-secocholesta-5, 7, 10-triene-3, 25-diol 10.83 508.280 0 279.233 0, 227.032 5, 152.995 0 ESI- -4.59 12 [STtrihydroxy(2∶0)]11beta_17_21-trihydroxypregn-4-ene-3_20-dione_21-sulfate 5.31 442.166 1 265.127 1, 222.107 7, 113.023 3 ESI- -2.83 13 {2-[2-(Isobutyryloxy)-4-methylphenyl]-2-oxiranyl}methyl 2-methylbutanoate 7.07 334.178 2 289.181 4, 245.191 5, 219.826 4 ESI- -2.42 14 1-(3, 4-Dimethoxyphenyl)-3, 5-decanediyl diacetate 3.34 394.235 9 242.919 2, 224.527 7, 153.091 4 ESI- 1.72 15 Histamine 19.56 111.080 0 / ESI+ 2.31 16 Cumyluron 3.05 302.118 8 254.852 7, 228.046 1, 79.956 1 ESI- -3.60 17 Hexahydrocurcumin 9.12 374.172 5 329.176 3, 285.186 4, 270.162 9, 229.123 5 ESI- -1.65 18 2-Phenylethyl D-glucopyranoside 2.44 284.126 0 265.108 3, 221.118 0, 203.107 3, 177.127 8 ESI- -1.17 19 Protoporphyrin Ⅸ 18.35 580.398 2 403.358 5, 113.023 3, 85.028 3 ESI- -1.38 20 3-Chlorotyrosine 1.86 215.034 5 / ESI- -2.07 21 4-(Heptyloxy)benzoic acid 4.07 236.140 9 191.143 7, 146.937 7, 103.919 2 ESI- -1.76 22 4-Allyl-2-methoxyphenyl 6-O-beta-D-xylopyranosyl-beta-D-glucopyranoside 2.60 458.179 3 266.098 2, 193.034 9, 113.023 3 ESI- -1.04 23 4-Hydroxy-N, N, N-trimethyl-9-oxo-7-[(propiony-loxy)methyl]-3, 5, 8-trioxa-4-phosphatetracosan-1-aminium 4-oxide 16.61 552.366 8 375.326 7, 220.545 2, 175.023 9, 113.023 3 ESI- -1.32 24 8'-Hydroxyabscisate 4.07 280.131 1 235.134 0, 191.143 7, 162.838 2 ESI- -1.63 25 Aderbasib 3.53 416.204 8 239.164 7, 193.035 2, 113.023 4 ESI- 1.01 26 Catalpol 3.93 362.122 3 313.111 6, 80.964 1 ESI- -2.76 27 Cholic acid 4.93 408.287 6 352.102 3, 220.830 4 ESI- -5.86 28 Tegobuvir 13.35 517.114 8 / ESI- -1.38 29 Deoxycholic acid 8.09 392.292 9 345.279 9, 221.415 0 ESI- -1.98 30 Helenalin 6.91 262.1205 235.134 0, 217.122 7, 191.143 5 ESI- -2.03 31 Hippuric acid 1.86 179.057 6 134.987 0, 106.934 7 ESI- -1.40 32 Isodomedin 4.13 392.220 1 305.805 8, 221.422 2, 164.835 6, 123.081 9, 78.427 5 ESI- 2.06 33 Lusitanicoside 3.90 442.184 0 193.035 0, 175.024 3, 113.023 4 ESI- -1.10 34 MFCD00273074 10.37 344.292 9 233.214 3, 136.908 9 ESI- -2.53 35 MFCD12546417 1.47 189.993 0 / ESI- -1.14 36 NBenzyloxycarbonyl-L-leucine 5.77 265.131 6 220.134 1, 192.139 1, 163.112 1, 82.028 9 ESI- 1.24 37 NP-005196 3.32 282.146 7 263.129 1, 237.149 5, 219.138 9 ESI- -1.41 38 NP-021701 6.02 266.151 9 247.134 0, 203.144 8, 191.144 0 ESI- -1.08 39 Taurodeoxycholic acid 5.90 499.296 8 397.434 5, 221.893 0 ESI- -2.10 40 Tetrofosmin 15.41 382.239 2 301.830 3, 96.959 1 ESI- 3.15 注: FC(SA high dose vs Model)。 -
[1] JANSEN P L M, GHALLAB A, VARTAK N, et al. The ascending pathophysiology of cholestatic liver disease[J]. Hepatology, 2017, 65(2): 722-738. doi: 10.1002/hep.28965 [2] DYSON J K, BEUERS U, JONES D E J, et al. Primary sclerosing cholangitis[J]. Lancet, 2018, 391(10139): 2547-2559. doi: 10.1016/S0140-6736(18)30300-3 [3] HSU C L, SCHNABL B. The gut-liver axis and gut microbiota in health and liver disease[J]. Nat Rev Microbiol, 2023, 21(11): 719-733. doi: 10.1038/s41579-023-00904-3 [4] SAHOO S M, MAHAPATRA S J. Intrahepatic cholestasis of pregnancy: Are we expecting too much from ursodeoxycholic acid?[J]. Lancet Gastroenterol Hepatol, 2021, 6(11): 886. [5] CHAPPELL L C, BELL J L, SMITH A, et al. Ursodeoxycholic acid versus placebo in women with intrahepatic cholestasis of pregnancy (PITCHES): A randomised controlled trial[J]. Lancet, 2019, 394(10201): 849-860. doi: 10.1016/S0140-6736(19)31270-X [6] ARUMUGAM B, BALAGANGADHARAN K, SELVAMURUGAN N. Syringic acid, a phenolic acid, promotes osteoblast differentiation by stimulation of Runx2 expression and targeting of Smad7 by miR-21 in mouse mesenchymal stem cells[J]. J Cell Commun Signal, 2018, 12(3): 561-573. doi: 10.1007/s12079-018-0449-3 [7] OGUT E, ARMAGAN K, GUL Z. The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations[J]. Metab Brain Dis, 2022, 37(4): 859-880. doi: 10.1007/s11011-022-00960-3 [8] LUO Q Q, GONG P F, SHI R Y, et al. Syringic acid alleviates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota[J]. J Agric Food Chem, 2023, 71(22): 8458-8470. doi: 10.1021/acs.jafc.3c02441 [9] GUZELAD O, OZKAN A, PARLAK H, et al. Protective mechanism of Syringic acid in an experimental model of Parkinson's disease[J]. Metab Brain Dis, 2021, 36(5): 1003-1014. doi: 10.1007/s11011-021-00704-9 [10] SRINIVASULU C, RAMGOPAL M, RAMANJANEYULU G, et al. Syringic acid (SA): A review of its occurrence, biosynthesis, pharmacological and industrial importance[J]. Biomed Pharmacother, 2018, 108: 547-557. doi: 10.1016/j.biopha.2018.09.069 [11] GHEENA S, EZHILARASAN D, SHREE HARINI K, et al. Syringic acid and silymarin concurrent administration inhibits sodium valproate-induced liver injury in rats[J]. Environ Toxicol, 2022, 37(9): 2143-2152. doi: 10.1002/tox.23557 [12] ITOH A, ISODA K, KONDOH M, et al. Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury[J]. Biol Pharm Bull, 2010, 33(6): 983-987. doi: 10.1248/bpb.33.983 [13] 吴守燕, 王雯婕, 颜晓霞, 等. α-萘异硫氰酸盐(ANIT)诱导的肝损伤作用研究[C]//中国毒理学会药物毒理与安全性评价学术大会(2019年)暨粤港澳大湾区生物医药产业第一届高峰论坛论文集. 广州, 2019: 334.WU S Y, WANG W J, YAN X X, etc. Study on the Liver Damage Induced by α-Naphthylisothiocyanate (ANIT)[C]// Academic conference on drug toxicology and safety evaluation of the Chinese toxicological society (2019) and the no. 1 biopharmaceutical industry in the Guangdong-Hong Kong-Macao greater bay area proceedings of the 2019 summit forum. Guangzhou, 2019: 334. [14] 吕超, 石清兰, 覃倩, 等. 小鼠实验性肝损伤模型的研究进展[J]. 中国比较医学杂志, 2019, 29(1): 107-113. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGDX201901019.htmLYU C, SHI Q L, QIN Q, et al. A review of experimental liver injury models in mice[J]. Chin J Comp Med, 2019, 29(1): 107-113. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGDX201901019.htm [15] OU Q Q, QIAN X H, LI D Y, et al. Yinzhihuang attenuates ANIT-induced intrahepatic cholestasis in rats through upregulation of Mrp2 and Bsep expressions[J]. Pediatr Res, 2016, 79(4): 589-595. doi: 10.1038/pr.2015.252 [16] THEILER-SCHWETZ V, ZAUFEL A, SCHLAGER H, et al. Bile acids and glucocorticoid metabolism in health and disease[J]. Biochim Biophys Acta Mol Basis Dis, 2019, 1865(1): 243-251. doi: 10.1016/j.bbadis.2018.08.001 [17] 李飞, 陆伦根. 肝功能异常的评估及临床意义[J]. 临床肝胆病杂志, 2015, 31(9): 1543-1546. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD201509060.htmLI F, LU L G. Evaluation of abnormal liver function and its clinical significance[J]. J Clin Hepatol, 2015, 31(9): 1543-1546. https://www.cnki.com.cn/Article/CJFDTOTAL-LCGD201509060.htm [18] TILG H, ADOLPH T E, TRAUNER M. Gut-liver axis: Pathophysiological concepts and clinical implications[J]. Cell Metab, 2022, 34(11): 1700-1718. doi: 10.1016/j.cmet.2022.09.017 [19] LORENZO-ZÚNIGA V, BARTOLI R, PLANAS R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats[J]. Hepatology, 2003, 37(3): 551-557. doi: 10.1053/jhep.2003.50116 [20] VERBEKE L, FARRE R, VERBINNEN B, et al. The FXR agonist obeticholic acid prevents gut barrier dysfunction and bacterial translocation in cholestatic rats[J]. Am J Pathol, 2015, 185(2): 409-419. doi: 10.1016/j.ajpath.2014.10.009 [21] ABRAHAM C, ABREU M T, TURNER J R. Pattern recognition receptor signaling and cytokine networks in microbial defenses and regulation of intestinal barriers: Implications for inflammatory bowel disease[J]. Gastroenterology, 2022, 162(6): 1602-1616. doi: 10.1053/j.gastro.2021.12.288 [22] MERLEN G, KAHALE N, URSIC-BEDOYA J, et al. TGR5-dependent hepatoprotection through the regulation of biliary epithelium barrier function[J]. Gut, 2020, 69(1): 146-157. doi: 10.1136/gutjnl-2018-316975 [23] SCHNEIDER K M, ALBERS S, TRAUTWEIN C. Role of bile acids in the gut-liver axis[J]. J Hepatol, 2018, 68(5): 1083-1085. doi: 10.1016/j.jhep.2017.11.025 -





 下载:
下载: