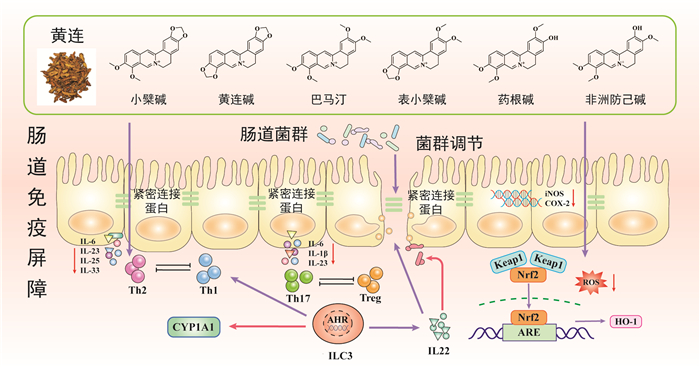
| [1] |
SINGH S, ANANTHAKRISHNAN AN, NGUYEN NH, et al. AGA clinical practice guideline on the role of biomarkers for the management of ulcerative colitis[J]. Gastroenterology, 2023, 164(3): 344-372. doi: 10.1053/j.gastro.2022.12.007
|
| [2] |
MAK JWY, LOK TUNG HO C, WONG K, et al. Epidemiology and natural history of elderly-onset inflammatory bowel disease: Results from a territory-wide IBD registry[J]. J Crohns Colitis, 2021, 15(3): 401-408. doi: 10.1093/ecco-jcc/jjaa181
|
| [3] |
LOPES EW, CHAN SSM, SONG MY, et al. Lifestyle factors for the prevention of inflammatory bowel disease[J]. Gut, 2022: gutjnl-gu2022-328174.
|
| [4] |
DIN S, SELINGER CP, BLACK CJ, et al. Systematic review with network meta-analysis: Risk of Herpes zoster with biological therapies and small molecules in inflammatory bowel disease[J]. Aliment Pharmacol Ther, 2023, 57(6): 666-675. doi: 10.1111/apt.17379
|
| [5] |
朱磊, 程成, 刘小娟, 等. 溃疡性结肠炎大肠湿热证研究现状与思考[J]. 南京中医药大学学报, 2023, 39(2): 188-193. doi: 10.14148/j.issn.1672-0482.2023.0188ZHU L, CHENG C, LIU XJ, et al. Research status and thinking of ulcerative colitis with large intestine damp-heat syndrome[J]. J Nanjing Univ Tradit Chin Med, 2023, 39(2): 188-193. doi: 10.14148/j.issn.1672-0482.2023.0188
|
| [6] |
林燕, 王新月, 韩昌盛. 中医综合治疗方案治疗溃疡性结肠炎的疗效评价[J]. 世界中西医结合杂志, 2010, 5(11): 956-958. doi: 10.13935/j.cnki.sjzx.2010.11.010LIN Y, WANG XY, HAN CS. Efficacy assessment of ulcerative colitis treated with comprehensive program of Chinese medicine[J]. World J Integr Tradit West Med, 2010, 5(11): 956-958. doi: 10.13935/j.cnki.sjzx.2010.11.010
|
| [7] |
YANG YH, KANG N, XIA HJ, et al. Metabolites of protoberberine alkaloids in human urine following oral administration of Coptidis Rhizoma Decoction[J]. Planta Med, 2010, 76(16): 1859-1863. doi: 10.1055/s-0030-1250053
|
| [8] |
HENRIKSEN M, JAHNSEN J, LYGREN I, et al. Are there any differences in phenotype or disease course between familial and sporadic cases of inflammatory bowel disease?Results of a population-based follow-up study[J]. Am J Gastroenterol, 2007, 102(9): 1955-1963. doi: 10.1111/j.1572-0241.2007.01368.x
|
| [9] |
ANANTHAKRISHNAN AN, NGUYEN DD, SAUK J, et al. Genetic polymorphisms in metabolizing enzymes modifying the association between smoking and inflammatory bowel diseases[J]. Inflamm Bowel Dis, 2014, 20(5): 783-789. doi: 10.1097/MIB.0000000000000014
|
| [10] |
BIEDERMANN L, ROGLER G. The intestinal microbiota: Its role in health and disease[J]. Eur J Pediatr, 2015, 174(2): 151-167. doi: 10.1007/s00431-014-2476-2
|
| [11] |
LIU YT, HAO HP, XIE HG, et al. Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats[J]. Drug Metab Dispos, 2010, 38(10): 1779-1784. doi: 10.1124/dmd.110.033936
|
| [12] |
MA BL, MA YM, SHI R, et al. Identification of the toxic constituents in Rhizoma Coptidis[J]. J Ethnopharmacol, 2010, 128(2): 357-364. doi: 10.1016/j.jep.2010.01.047
|
| [13] |
LI Q, CHEN ZH, ZHANG JR, et al. The colonic metabolism differences of main alkaloids in normal and colitis mice treated with Coptis chinensis Franch. and Sophora flavescens Ait. herbal pair using liquid chromatography-high resolution mass spectrometry method combined with chemometrics[J]. J Sep Sci, 2023, 46(14): e2300094. doi: 10.1002/jssc.202300094
|
| [14] |
YANG YH, KANG N, XIA HJ, et al. Metabolites of protoberberine alkaloids in human urine following oral administration of Coptidis Rhizoma Decoction[J]. Planta Med, 2010, 76(16): 1859-1863. doi: 10.1055/s-0030-1250053
|
| [15] |
ZUO F, NAKAMURA N, AKAO T, et al. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry[J]. Drug Metab Dispos, 2006, 34(12): 2064-2072. doi: 10.1124/dmd.106.011361
|
| [16] |
GONG ZP, CHEN Y, ZHANG RJ, et al. Pharmacokinetic comparison of berberine in rat plasma after oral administration of berberine hydrochloride in normal and post inflammation irritable bowel syndrome rats[J]. Int J Mol Sci, 2014, 15(1): 456-467. doi: 10.3390/ijms15010456
|
| [17] |
XUE BJ, ZHAO YY, MIAO Q, et al. In vitro and in vivo identification of metabolites of magnoflorine by LC LTQ-Orbitrap MS and its potential pharmacokinetic interaction in Coptidis Rhizoma Decoction in rat[J]. Biomed Chromatogr, 2015, 29(8): 1235-1248. doi: 10.1002/bmc.3413
|
| [18] |
WANG K, FENG XC, CHAI LW, et al. The metabolism of berberine and its contribution to the pharmacological effects[J]. Drug Metab Rev, 2017, 49(2): 139-157. doi: 10.1080/03602532.2017.1306544
|
| [19] |
AI GX, HUANG ZW, CHENG JJ, et al. Gut microbiota-mediated transformation of coptisine into a novel metabolite 8-oxocoptisine: Insight into its superior anti-colitis effect[J]. Front Pharmacol, 2021, 12: 639020. doi: 10.3389/fphar.2021.639020
|
| [20] |
WANG B, GONG Z, ZHAN J, et al. Xianglian pill suppresses inflammation and protects intestinal epithelial barrier by promoting autophagy in DSS induced ulcerative colitis mice[J]. Front Pharmacol, 2020, 11: 594847.
|
| [21] |
YANG YM, HUA YW, CHEN WH, et al. Therapeutic targets and pharmacological mechanisms of Coptidis Rhizoma against ulcerative colitis: Findings of system pharmacology and bioinformatics analysis[J]. Front Pharmacol, 2022, 13: 1037856. doi: 10.3389/fphar.2022.1037856
|
| [22] |
LEE IA, HYUN YJ, KIM DH. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation[J]. Eur J Pharmacol, 2010, 648(1/2/3): 162-170.
|
| [23] |
CHENG JJ, MA XD, AI GX, et al. Palmatine protects against MSU-induced gouty arthritis via regulating the NF-κB/NLRP3 and Nrf2 pathways[J]. Drug Des Devel Ther, 2022, 16: 2119-2132. doi: 10.2147/DDDT.S356307
|
| [24] |
ZHENG YN, SHI X, HOU JB, et al. Integrating metabolomics and network pharmacology to explore Rhizoma Coptidis extracts against sepsis-associated acute kidney injury[J]. J Chromatogr B Analyt Technol Biomed Life Sci, 2021, 1164: 122525. doi: 10.1016/j.jchromb.2021.122525
|
| [25] |
YUAN HY, WANG BH, YE ZC, et al. Berberine alleviates the damage, oxidative stress and mitochondrial dysfunction of PC12 cells induced by high glucose by activating the KEAP1/Nrf2/ARE pathway[J]. Mol Biotechnol, 2023, 65(10): 1632-1643. doi: 10.1007/s12033-022-00651-5
|
| [26] |
JING WH, SAFARPOUR Y, ZHANG T, et al. Berberine upregulates P-glycoprotein in human caco-2 cells and in an experimental model of colitis in the rat via activation of Nrf2-dependent mechanisms[J]. J Pharmacol Exp Ther, 2018, 366(2): 332-340. doi: 10.1124/jpet.118.249615
|
| [27] |
LI CL, LIU MG, DENG L, et al. Oxyberberine ameliorates TNBS-induced colitis in rats through suppressing inflammation and oxidative stress via Keap1/Nrf2/NF-κB signaling pathways[J]. Phytomedicine, 2023, 116: 154899. doi: 10.1016/j.phymed.2023.154899
|
| [28] |
FU KQ, WANG ZZ, CAO RF. Berberine attenuates the inflammatory response by activating the Keap1/Nrf2 signaling pathway in bovine endometrial epithelial cells[J]. Int Immunopharmacol, 2021, 96: 107738. doi: 10.1016/j.intimp.2021.107738
|
| [29] |
SHOU JW, LI XX, TANG YS, et al. Novel mechanistic insight on the neuroprotective effect of berberine: The role of PPARδ for antioxidant action[J]. Free Radic Biol Med, 2022, 181: 62-71. doi: 10.1016/j.freeradbiomed.2022.01.022
|
| [30] |
PEI H, ZENG J, HE Z, et al. Palmatine ameliorates LPS-induced HT-22 cells and mouse models of depression by regulating apoptosis and oxidative stress[J]. J Biochem Mol Toxicol, 2023, 37(1): e23225. doi: 10.1002/jbt.23225
|
| [31] |
CHENG JJ, MA XD, ZHANG HT, et al. 8-Oxypalmatine, a novel oxidative metabolite of palmatine, exhibits superior anti-colitis effect via regulating Nrf2 and NLRP3 inflammasome[J]. Biomed Pharmacother, 2022, 153: 113335. doi: 10.1016/j.biopha.2022.113335
|
| [32] |
LI N, GU LL, QU LL, et al. Berberine attenuates pro-inflammatory cytokine-induced tight junction disruption in an in vitro model of intestinal epithelial cells[J]. Eur J Pharm Sci, 2010, 40(1): 1-8. doi: 10.1016/j.ejps.2010.02.001
|
| [33] |
DIGUILIO KM, MERCOGLIANO CM, BORN J, et al. Sieving characteristics of cytokine- and peroxide-induced epithelial barrier leak: Inhibition by berberine[J]. World J Gastrointest Pathophysiol, 2016, 7(2): 223-234. doi: 10.4291/wjgp.v7.i2.223
|
| [34] |
YAN F, WANG LH, SHI Y, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice[J]. Am J Physiol Gastrointest Liver Physiol, 2012, 302(5): G504-G514. doi: 10.1152/ajpgi.00312.2011
|
| [35] |
HAO XH, YAO AL, GONG JF, et al. Berberine ameliorates pro-inflammatory cytokine-induced endoplasmic reticulum stress in human intestinal epithelial cells in vitro[J]. Inflammation, 2012, 35(3): 841-849. doi: 10.1007/s10753-011-9385-6
|
| [36] |
KEMPSKI J, BROCKMANN L, GAGLIANI N, et al. TH17 cell and epithelial cell crosstalk during inflammatory bowel disease and carcinogenesis[J]. Front Immunol, 2017, 8: 1373. doi: 10.3389/fimmu.2017.01373
|
| [37] |
LI YH, XIAO HT, HU DD, et al. Berberine ameliorates chronic relapsing dextran sulfate sodium-induced colitis in C57BL/6 mice by suppressing Th17 responses[J]. Pharmacol Res, 2016, 110: 227-239. doi: 10.1016/j.phrs.2016.02.010
|
| [38] |
CUI HT, CAI YZ, WANG L, et al. Berberine regulates treg/Th17 balance to treat ulcerative colitis through modulating the gut microbiota in the colon[J]. Front Pharmacol, 2018, 9: 571. doi: 10.3389/fphar.2018.00571
|
| [39] |
LI H, FAN C, LU HM, et al. Protective role of berberine on ulcerative colitis through modulating enteric glial cells-intestinal epithelial cells-immune cells interactions[J]. Acta Pharm Sin B, 2020, 10(3): 447-461.
|
| [40] |
LIU YX, LIU X, HUA WW, et al. Berberine inhibits macrophage M1 polarization via AKT1/SOCS1/NF-κB signaling pathway to protect against DSS-induced colitis[J]. Int Immunopharmacol, 2018, 57: 121-131.
|
| [41] |
ZINDL CL, WITTE SJ, LAUFER VA, et al. A nonredundant role for T cell-derived interleukin 22 in antibacterial defense of colonic crypts[J]. Immunity, 2022, 55(3): 494-511. e11.
|
| [42] |
MICHAUDEL C, DANNE C, AGUS A, et al. Rewiring the altered tryptophan metabolism as a novel therapeutic strategy in inflammatory bowel diseases[J]. Gut, 2023, 72(7): 1296-1307.
|
| [43] |
WANG XM, LIANG FN, DAI ZY, et al. Combination of Coptis chinensis polysaccharides and berberine ameliorates ulcerative colitis by regulating gut microbiota and activating AhR/IL-22 pathway[J]. J Ethnopharmacol, 2024, 318(Pt B): 117050.
|
| [44] |
SONG MM, SHENG XJ, ZHANG JR, et al. Homeostatic regulation of the aryl hydrocarbon receptor-cytochrome P450 1a axis by Scutellaria baicalensis-Coptis chinensis herb pair and its main constituents[J]. J Ethnopharmacol, 2022, 297: 115545.
|
| [45] |
ZHOU R, HUANG YY, TIAN CJ, et al. Coptis chinensis and berberine ameliorate chronic ulcerative colitis: An integrated microbiome-metabolomics study[J]. Am J Chin Med, 2023: 1-26.
|
| [46] |
JING WH, DONG SJ, LUO XL, et al. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites[J]. Pharmacol Res, 2021, 164: 105358.
|
| [47] |
SUN XJ, ZHANG Y, CHENG G, et al. Berberine improves DSS-induced colitis in mice by modulating the fecal-bacteria-related bile acid metabolism[J]. Biomed Pharmacother, 2023, 167: 115430.
|




 下载:
下载: