Effects of Four Commonly Used Chinese Medicines with Clearing Heat and Drying Dampness on Intestinal Flora Mediated Bile Acids and Short Chain Fatty Acids Metabolism
-
摘要:
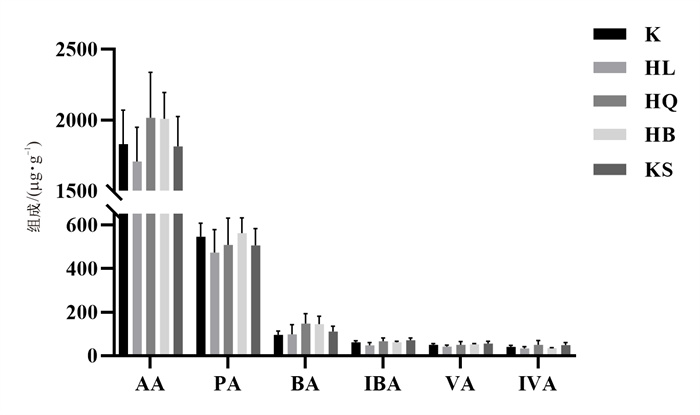
目的 探究在长期临床等效剂量下4种清热燥湿类中药对正常小鼠肠道菌群、胆汁酸及短链脂肪酸的影响。 方法 30只Balb/c雄性小鼠被随机分为对照组、苦参组、黄连组、黄柏组和黄芩组, 每组各6只。除对照组外, 苦参组、黄柏组、黄连组和黄芩组给药剂量分别为2.34、3.12、1.3 g·kg-1和2.6 g·kg-1, 连续灌胃2周, 采用液相色谱-串联质谱法检测粪便中短链脂肪酸和胆汁酸含量, 利用16S rRNA高通量基因测序技术分析肠道内容物中菌群结构变化。 结果 清热燥湿类中药对正常小鼠粪便中6种短链脂肪酸无明显影响。在常见的23种胆汁酸中, 与正常组相比, 黄芩组、苦参组、黄连组和黄柏组分别有4、3、2、1种胆汁酸显著变化。从肠道菌群丰度变化上看, 与对照组相比, 除苦参组外, 黄连组、黄芩组和黄柏组小鼠厚壁菌门均呈现下降趋势, 拟杆菌门均呈现上升趋势, 其中黄连组变化更显著。 结论 苦参、黄柏、黄连和黄芩对正常小鼠长期药效剂量下短链脂肪酸影响较小, 但可以通过改变肠道菌群结构影响胆汁酸含量的变化。 Abstract:OBJECTIVE To explore the effects of four kinds of heat-clearing and dampness-drying herbs on intestinal flora, bile acids and short chain fatty acids (SCFAs) in normal mice at long-term clinical equivalent doses. METHODS Thirty Balb/c male mice were randomly divided into control group, Sophora flavescens Ait. (KS) group, Phellodendron chinense Schneid. (HB) group, Coptis chinensis Franch. (HL) group and Scutellaria baicalensis Georgi (HQ) group, with 6 mice in each group. Except for the control group, KS group, HB group, HL group and HQ group were orally administrated at a dosage of 2.34 g·kg-1, 3.12 g·kg-1, 1.3 g·kg-1 and 2.6 g·kg-1, respectively, for two weeks. The contents of short chain fatty acids and bile acids in feces were detected by liquid chromatography tandem mass spectrometry, and the structural changes of microflora in intestinal contents were detected by 16S rRNA high-throughput gene sequencing technology. RESULTS There was no significant effect of heat-clearing and dampness-drying Chinese medicine on the contents of six short-chain fatty acids in the feces of normal mice. Among the 23 common bile acids, when compared with the control group, the numbers of the significantly changed bile acids in HQ group, KS group, HL group and HB group were 4, 3, 2 and 1, respectively. From the perspective of changes in the abundance of intestinal flora, compared with the control group, except for the KS group, Firmicutes in the HL group, HQ group and HB group all showed a downward trend, and the Bacteroides showed an upward trend, among which the changes in the HL group were the most significant. CONCLUSION Sophora flavescens Ait., Phellodendron chinense Schneid., Coptis chinensis Franch. and Scutellaria baicalensis Georgi have little effect on SCFAs in normal mice, but can affect bile acid content by changing the structure of intestinal flora. -
表 1 胆汁酸及内标的LC-MS/MS参数
Table 1. LC-MS/MS parameters for bile acids and internal standard
ID Q1 Q3 DP/V CE/eV CXP LCA 375.300 375.300 -210.000 -10.000 -10.000 DCA、HDCA、UDCA、CDCA 391.300 391.300 -230.000 -10.000 -10.000 Dehydro-CA 401.200 401.200 -200.000 -10.000 -10.000 CA、α-MCA、β-MCA、ω-MAC 407.200 407.200 -245.000 -10.000 -10.000 GUDCA、GCDCA、GDCA、HDCA 448.200 74.000 -210.000 -83.000 -9.000 GCA 464.200 74.000 -170.000 -88.000 -11.000 TDCA、THDCA、TUDCA、TCDCA 498.200 80.000 -280.000 -130.000 -9.000 TCA、T-α-MCA 514.200 80.000 -205.000 -130.000 -9.000 CDCA-d4 395.200 359.200 -210.000 -10.000 -10.000 GCA-d4 468.200 74.000 -170.000 -88.000 -11.000 表 2 黄连、黄芩、黄柏和苦参组小鼠肠道菌群α多样性指数比较(x±s)
Table 2. Comparison of intestinal flora α diversity indices of mice in HL, HQ, HB and KS groups(x±s)
组别 ACE Chao Shannon Simpson C 401.18±80.85 405.41±86.75 3.67±0.49 0.07±0.05 HB 353.61±18.4 360.17±15.31 3.52±0.32 0.08±0.04 HL 255.31±28.56**** 257.02±27.55**** 2.9±0.38** 0.13±0.05 HQ 376.42±54.55 379.69±53.43 3.81±0.23 0.04±0.01 KS 455.58±26.99 470.4±30.29 3.73±0.49 0.08±0.05 注: 与C组比较,**P < 0.01, ****P < 0.000 1。 -
[1] ADAK A, KHAN MR. An insight into gut microbiota and its functionalities[J]. Cell Mol Life Sci, 2019, 76(3): 473-493. doi: 10.1007/s00018-018-2943-4 [2] SINGH RK, CHANG HW, YAN D, et al. Influence of diet on the gut microbiome and implications for human health[J]. J Transl Med, 2017, 15(1): 73. doi: 10.1186/s12967-017-1175-y [3] SCHOELER M, CAESAR R. Dietary lipids, gut microbiota and lipid metabolism[J]. Rev Endocr Metab Disord, 2019, 20(4): 461-472. doi: 10.1007/s11154-019-09512-0 [4] 牛璐, 王跃飞, 赵鑫, 等. 中药调控肠道菌群代谢产物的研究进展[J]. 天津中医药, 2021, 38(2): 254-260. https://www.cnki.com.cn/Article/CJFDTOTAL-TJZY202102029.htmNIU L, WANG YF, ZHAO X, et al. Research progress on the regulation of gut microbial metabolites by traditional Chinese medicine[J]. J Tianjin Univ Tradit Chin Med, 2021, 38(2): 254-260. https://www.cnki.com.cn/Article/CJFDTOTAL-TJZY202102029.htm [5] RATAJCZAK W, RYŁ A, MIZERSKI A, et al. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs)[J]. Acta Biochim Pol, 2019, 66(1): 1-12. [6] YANG WJ, YU TM, HUANG XS, et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity[J]. Nat Commun, 2020, 11(1): 4457. doi: 10.1038/s41467-020-18262-6 [7] WANG G, YU Y, WANG YZ, et al. Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy[J]. J Cell Physiol, 2019, 234(10): 17023-17049. doi: 10.1002/jcp.28436 [8] PORTINCASA P, BONFRATE L, VACCA M, et al. Gut microbiota and short chain fatty acids: Implications in glucose homeostasis[J]. Int J Mol Sci, 2022, 23(3): 1105. doi: 10.3390/ijms23031105 [9] HUANG W, MAN Y, GAO CL, et al. Short-chain fatty acids ameliorate diabetic nephropathy via GPR43-mediated inhibition of oxidative stress and NF-κB signaling[J]. Oxid Med Cell Longev, 2020, 2020: 4074832. [10] COLOSIMO S, TOMLINSON JW. Bile acids as drivers and biomarkers of hepatocellular carcinoma[J]. World J Hepatol, 2022, 14(9): 1730-1738. doi: 10.4254/wjh.v14.i9.1730 [11] LI JN, DAWSON PA. Animal models to study bile acid metabolism[J]. Biochim Biophys Acta Mol Basis Dis, 2019, 1865(5): 895-911. doi: 10.1016/j.bbadis.2018.05.011 [12] WINSTON JA, THERIOT CM. Diversification of host bile acids by members of the gut microbiota[J]. Gut Microbes, 2020, 11(2): 158-171. doi: 10.1080/19490976.2019.1674124 [13] MCKENZIE C, TAN J, MACIA L, et al. The nutrition-gut microbiome-physiology axis and allergic diseases[J]. Immunol Rev, 2017, 278(1): 277-295. doi: 10.1111/imr.12556 [14] FIORUCCI S, CARINO A, BALDONI M, et al. Bile acid signaling in inflammatory bowel diseases[J]. Dig Dis Sci, 2021, 66(3): 674-693. doi: 10.1007/s10620-020-06715-3 [15] DELEU S, MACHIELS K, RAES J, et al. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD?[J]. EBioMedicine, 2021, 66: 103293. doi: 10.1016/j.ebiom.2021.103293 [16] WANG J, WANG L, LOU GH, et al. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology[J]. Pharm Biol, 2019, 57(1): 193-225. doi: 10.1080/13880209.2019.1577466 [17] SUN Y, LENON GB, YANG AWH. Phellodendri cortex: A phytochemical, pharmacological, and pharmacokinetic review[J]. Evid Based Complement Alternat Med, 2019, 2019: 7621929. [18] WANG ZL, WANG S, KUANG Y, et al. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis[J]. Pharm Biol, 2018, 56(1): 465-484. doi: 10.1080/13880209.2018.1492620 [19] LI X, TANG ZW, WEN L, et al. Matrine: A review of its pharmacology, pharmacokinetics, toxicity, clinical application and preparation researches[J]. J Ethnopharmacol, 2021, 269: 113682. doi: 10.1016/j.jep.2020.113682 [20] PAN LL, LI ZZ, WANG YF, et al. Network pharmacology and metabolomics study on the intervention of traditional Chinese medicine Huanglian Decoction in rats with type 2 diabetes mellitus[J]. J Ethnopharmacol, 2020, 258: 112842. doi: 10.1016/j.jep.2020.112842 [21] 章常华, 魏悦, 施旻, 等. 知母黄柏药对对肥胖症大鼠模型降脂作用的实验研究[J]. 时珍国医国药, 2021, 32(4): 773-776.ZHANG CH, WEI Y, SHI M, et al. Experimental study on the lipid-lowering effect of Anemarrhenae Rhizoma-Phelloden-dri Chinensis Cortex herb pair on obesity rat model[J]. Lishizhen Med Mater Med Res, 2021, 32(4): 773-776. [22] XIAO SW, LIU C, CHEN MJ, et al. Scutellariae radix and coptidis rhizoma ameliorate glycolipid metabolism of type 2 diabetic rats by modulating gut microbiota and its metabolites[J]. Appl Microbiol Biotechnol, 2020, 104(1): 303-317. doi: 10.1007/s00253-019-10174-w [23] SHAO J, LIU Y, WANG H, et al. An integrated fecal microbiome and metabolomics in T2DM rats reveal antidiabetes effects from host-microbial metabolic axis of EtOAc extract from Sophora flavescens[J]. Oxid Med Cell Longev, 2020, 2020: 1805418. [24] WANG BT, KONG QM, LI X, et al. A high-fat diet increases gut microbiota biodiversity and energy expenditure due to nutrient difference[J]. Nutrients, 2020, 12(10): 3197. doi: 10.3390/nu12103197 [25] YUAN XY, XUE J, TAN YX, et al. Albuca bracteate polysaccharides synergistically enhance the anti-tumor efficacy of 5-fluorouracil against colorectal cancer by modulating β-catenin signaling and intestinal flora[J]. Front Pharmacol, 2021, 12: 736627. doi: 10.3389/fphar.2021.736627 [26] KIM KH, PARK D, JIA BL, et al. Identification and characterization of major bile acid 7α-dehydroxylating bacteria in the human gut[J]. mSystems, 2022, 7(4): e0045522. doi: 10.1128/msystems.00455-22 [27] ZHAO H, GAO X, LIU ZZ, et al. Sodium alginate prevents non-alcoholic fatty liver disease by modulating the gut-liver axis in high-fat diet-fed rats[J]. Nutrients, 2022, 14(22): 4846. doi: 10.3390/nu14224846 [28] 黄玉普, 吴大章, 王森. 黄芩的药理作用及其药对研究进展[J]. 中国药业, 2022, 31(15): 1-5. https://www.cnki.com.cn/Article/CJFDTOTAL-YYGZ202215030.htmHUANG YP, WU DZ, WANG S. Research progress on pharmacological action of scutellariae Radix and its drug pair[J]. China Pharm, 2022, 31(15): 1-5. https://www.cnki.com.cn/Article/CJFDTOTAL-YYGZ202215030.htm [29] 彭程程, 姚亮亮, 曾国威, 等. 葛根芩连汤干预胰岛素抵抗大鼠的粪便代谢组及相关菌群研究[J]. 中药新药与临床药理, 2021, 32(12): 1737-1744.PENG CC, YAO LL, ZENG GW, et al. Study on fecal metabolic mechanism and related flora of Gegen Qinlian Decoction in rats with insulin resistance[J]. Tradit Chin Drug Res Clin Pharmacol, 2021, 32(12): 1737-1744. [30] ZHAO LJ, MA P, PENG Y, et al. Amelioration of hyperglycaemia and hyperlipidaemia by adjusting the interplay between gut microbiota and bile acid metabolism: Radix Scutellariae as a case[J]. Phytomedicine, 2021, 83: 153477. doi: 10.1016/j.phymed.2021.153477 [31] FANG YK, YAN C, ZHAO Q, et al. The roles of microbial products in the development of colorectal cancer: A review[J]. Bioengineered, 2021, 12(1): 720-735. doi: 10.1080/21655979.2021.1889109 [32] GOOSSENS JF, BAILLY C. Ursodeoxycholic acid and cancer: From chemoprevention to chemotherapy[J]. Pharmacol Ther, 2019, 203: 107396. -





 下载:
下载: