Study on the Effect and Mechanism of Danggui Buxue Decoction Inhibiting HK-2 Cell Fibrosis through miR-27a/TGF-β1/Smad3 Pathway
-
摘要:
目的 探讨当归补血汤通过miR-27a调控HK-2细胞纤维化的机制。 方法 HK-2细胞分为对照组、高糖组、高糖+空白血清组、高糖+当归补血汤含药血清组。采用MTT法检测各组细胞增殖情况; 采用Western blot法检测纤维化相关因子波形蛋白(Vimentin)、Ⅳ型胶原(COL Ⅳ)、α-平滑肌肌动蛋白(α-SMA)、p-Smad3/Smad3和转化生长因子(TGF)-β1蛋白的表达情况; qPCR法检测miR-27a及纤维化相关因子mRNA的表达情况; 通过转染miR-27a抑制剂及Smad3-siRNA并向培养基中加入TGF-β1, 观察miR-27a的表达和纤维化相关蛋白表达的情况。 结果 MTT结果显示, 与对照组相比, 高糖组细胞增殖率明显降低(P < 0.05, P < 0.001), 当归补血汤含药血清作用24、48 h后细胞增殖率明显提高(P < 0.05, P < 0.001)。Western blot结果显示, 当归补血汤含药血清明显抑制高糖诱导的HK-2细胞纤维化相关蛋白COL Ⅳ、α-SMA、Vimentin、TGF-β1、p-Smad3/Smad3的表达(P < 0.05, P < 0.01)。qPCR结果显示, 当归补血汤含药血清明显抑制高糖诱导的HK-2细胞COL Ⅳ、α-SMA、Vimentin、TGF-β1 mRNA和miR-27a的表达(P < 0.05, P < 0.01)。TGF-β1处理细胞后, 细胞纤维化加重, miR-27a的表达升高(P < 0.05, P < 0.01), 当归补血汤含药血清可明显降低TGF-β1诱导的纤维化细胞中miR-27a的表达升高(P < 0.001)。转染miR-27a抑制剂及Smad3-siRNA可明显降低TGF-β1诱导的细胞纤维化蛋白COL Ⅳ、α-SMA、Vimentin、TGF-β1、p-Smad3/Smad3表达的升高(P < 0.05, P < 0.01)。 结论 当归补血汤含药血清可促进HK-2细胞增殖, 并通过miR-27a/TGF-β1/Smad3信号通路调控HK-2细胞纤维化。 -
关键词:
- 当归补血汤 /
- HK-2 /
- miR-27a /
- TGF-β1/Smad3 /
- 纤维化
Abstract:OBJECTIVE To investigate the mechanism of Danggui Buxue Decoction regulating HK-2 cell fibrosis through miR-27a. METHODS HK-2 cells were divided into control group, high glucose group, high glucose+blank serum group, and high glucose+Danggui Buxue Decoction-containing serum group. The proliferation of cells in each group was detected by MTT assay; The expression of the fibrosis-related factors Vimentin, COL Ⅳ, α-SMA, p-Smad3/Smad3 and TGF-β1 protein was measured by Western blot; The qPCR was used to detect the expression of miR-27a and fibrosis-related factors mRNA; The expression of miR-27a and fibrosis-related proteins were observed by transfecting miR-27a inhibitor and Smad3-siRNA, as well as adding TGF-β1 to the culture medium. RESULTS MTT results showed that compared with the control group, the cell proliferation rate in the high glucose group significantly decreased (P < 0.05, P < 0.001), and Danggui Buxue Decoction significantly promoted cell proliferation (P < 0.05, P < 0.001). Western blot showed that Danggui Buxue Decoction significantly inhibited the expression of fibrosis-related proteins COL Ⅳ, α-SMA, Vimentin, TGF-β1 and p-Smad3/Smad3 in HK-2 cells induced by high glucose (P < 0.05, P < 0.01). The qPCR results were consistent with Western blot results. After TGF-β1 treatment of cells, cell fibrosis aggravated, and the expression of miR-27a increased (P < 0.05, P < 0.01), Danggui Buxue Decoction significantly reduced the elevation of miR-27a in TGF-β1-induced cellular fibrosis (P < 0.001). Transfection of miR-27a inhibitor and Smad3-siRNA could significantly reduce the increase of TGF-β1-induced cellular fibrosis protein expression (P < 0.05, P < 0.01). CONCLUSION Danggui Buxue Decoction-containing serum can promote the proliferation of HK-2 cells and regulate HK-2 cell fibrosis through miR-27a/TGF-β1/Smad3 signaling pathway. -
Key words:
- Danggui Buxue Decoction /
- HK-2 /
- miR-27a /
- TGF-β1/Smad3 /
- fibrosis
-
图 2 转染Smad3-siRNA及miR-27a抑制剂对TGF-β1处理后HK-2细胞纤维化相关蛋白表达的影响
注: anti-miR-27a.miR-27a抑制剂组; miR-Ctrl.miR-27a抑制剂阴性对照组; Smad3-siRNA.Smad3-siRNA组; Con-siRNA.Smad3-siRNA阴性对照组。组间比较, *P < 0.05, **P < 0.01, ***P < 0.001。
Figure 2. Transfection of Smad3-siRNA and miR-27a inhibitor on the expression of fibrosis-related proteins in HK-2 cells after TGF-β1 treatment
表 1 qPCR引物序列
Table 1. Primer sequences for qPCR
基因 正向引物 反向引物 TGF-β1 5'-GCGTCTAATGGTGGAAACC-3' 5'-GAGCAACACGGGTTCAGGTA-3' Vimentin 5'-CAGCATGGACGTTCGTCTG-3' 5'-AACCACGGTITGGTCCTTGG-3' α-SMA 5'-GTGTGTGTGTCTTGGTAATGG-3' 5'-CCTTGTGTCCTGATCGTTG-3' COL Ⅳ 5'-GAAGACATCCCACCAATCAC-3' 5'-CACGTCATCGCACAACAC-3' β-actin 5'-GGGACCTGACTGACTACCTC-3' 5'-TCATACTCCTGCTTGCTGAT-3' miR-27a 5'-GCGCGTTCACAGTGGCTAAG-3' 5'-AGTGCAGGGTCCGAGGTATT-3' U6 5'-CTCGCTTCGGCAGCACA-3' 5'-AACGCTTCACGAATTTGCGT-3' 表 2 当归补血汤含药血清对HK-2细胞增殖率的影响(x±s, %, n=6)
Table 2. Effect of Danggui Buxue Decoction (DBD) containing serum on HK-2 proliferative rate (x±s, %, n=6)
组别 12 h 24 h 48 h 对照组 100.00±21.72 100.00±18.10 100.00±13.19 高糖组 68.99±19.31* 48.16±18.84*** 52.84±16.66*** 高糖+空白血清组 72.75±14.66* 62.72±16.42** 58.57±8.56*** 高糖+含药血清组 85.40±19.99 81.09±15.55# 91.89±15.48### 注: 与对照组比较,*P < 0.05, **P < 0.01, ***P < 0.001;与高糖+空白血清组比较,#P < 0.05, ###P < 0.001。 表 3 当归补血汤对HK-2细胞纤维化相关蛋白表达的影响(x±s, n=3)
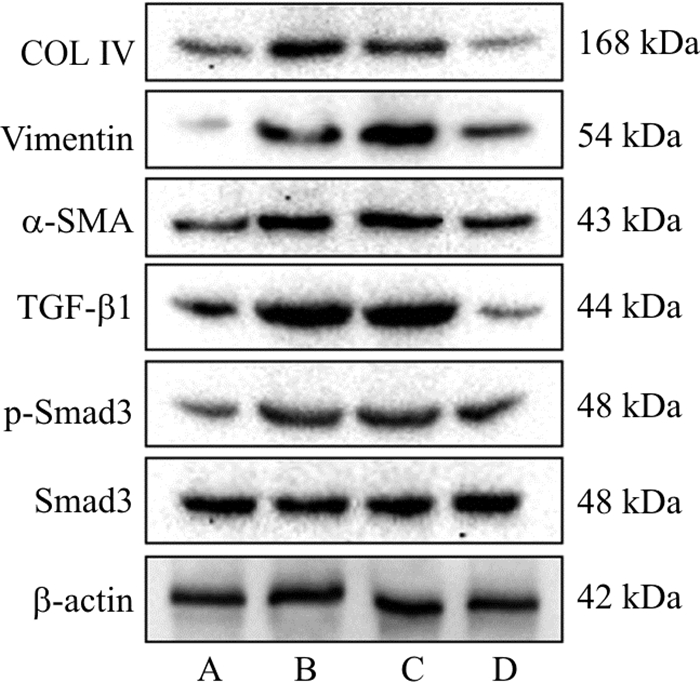
Table 3. Effect of DBD containing serum on the expression of fibrosis-related proteins in HK-2 cells (x±s, n=3)
组别 COL Ⅳ/β-actin Vimentin/β-actin α-SMA/β-actin TGF-β1/β-actin p-Smad3/Smad3 对照组 1.00±0.06 1.00±0.17 1.00±0.14 1.00±0.09 1.00±0.09 高糖组 2.17±0.17*** 1.99±0.13** 1.90±0.13** 2.55±0.08*** 1.59±0.21* 高糖+空白血清组 1.98±0.14** 2.30±0.25** 2.02±0.11*** 2.48±0.09*** 1.58±0.21* 高糖+含药血清组 1.07±0.19## 1.50±0.18# 1.56±0.10## 1.09±0.28## 1.21±0.08# 注: 与对照组比较, *P < 0.05, **P < 0.01, ***P < 0.001;与高糖+空白血清组比较, #P < 0.05, ##P < 0.01。 表 4 当归补血汤对HK-2细胞纤维化相关mRNA表达的影响(x±s, n=3)
Table 4. Effect of DBD containing serum on the expression of fibrosis-related mRNA in HK-2 cells (x±s, n=3)
组别 COL Ⅳ/β-actin Vimentin/β-actin α-SMA/β-actin TGF-β1/β-actin miR-27a/U6 对照组 1.00±0.13 1.00±0.10 1.00±0.14 1.00±0.06 1.00±0.10 高糖组 1.94±0.40* 1.30±0.06* 1.48±0.12* 1.58±0.07*** 1.47±0.13** 高糖+空白血清组 1.83±0.15** 1.41±0.07** 1.63±0.21* 1.80±0.40* 2.10±0.19*** 高糖+含药血清组 1.44±0.20## 1.21±0.07## 1.15±0.14# 1.09±0.22# 1.16±0.22## 注: 与对照组比较,*P < 0.05, **P < 0.01, ***P < 0.001;与高糖+空白血清组比较,#P < 0.05, ##P < 0.01。 表 5 TGF-β1处理HK-2细胞对miR-27a表达的影响(x±s, n=3)
Table 5. The effect of TGF-β1 treatment on the expression of miR-27a in HK-2 cells (x±s, n=3)
组别 miR-27a/U6 对照组 1.00±0.04 5 ng·mL-1 TGF-β1组 2.23±0.69* 10 ng·mL-1 TGF-β1组 4.09±0.63** 注: 与对照组比较, *P < 0.05, **P < 0.01。 表 6 TGF-β1及当归补血汤含药血清处理HK-2细胞对miR-27a表达的影响(x±s, n=3)
Table 6. The effect of TGF-β1 and DBD containing serum on the expression of miR-27a in HK-2 cells (x±s, n=3)
组别 miR-27a/U6 对照组 1.00±0.01 当归补血汤组 0.33±0.08*** 注: 与对照组比较, ***P < 0.001。 -
[1] AVOGARO A, FADINI GP. Microvascular complications in diabetes: A growing concern for cardiologists[J]. Int J Cardiol, 2019, 291: 29-35. doi: 10.1016/j.ijcard.2019.02.030 [2] YANG SF, ABDULLA R, LU C, et al. Inhibition of microRNA-376b protects against renal interstitial fibrosis via inducing macrophage autophagy by upregulating Atg5 in mice with chronic kidney disease[J]. Kidney Blood Press Res, 2018, 43(6): 1749-1764. doi: 10.1159/000495394 [3] TANG FJ, HAO YR, ZHANG X, et al. Effect of echinacoside on kidney fibrosis by inhibition of TGF-β1/Smads signaling pathway in the db/db mice model of diabetic nephropathy[J]. Drug Des Devel Ther, 2017, 11: 2813-2826. doi: 10.2147/DDDT.S143805 [4] WU LN, WANG QZ, GUO F, et al. Involvement of miR-27a-3p in diabetic nephropathy via affecting renal fibrosis, mitochondrial dysfunction, and endoplasmic Reticulum stress[J]. J Cell Physiol, 2021, 236(2): 1454-1468. doi: 10.1002/jcp.29951 [5] HOU XY, TIAN JW, GENG J, et al. microRNA-27a promotes renal tubulointerstitial fibrosis via suppressing PPARγ pathway in diabetic nephropathy[J]. Oncotarget, 2016, 7(30): 47760-47776. doi: 10.18632/oncotarget.10283 [6] SHI XQ, YUE SJ, TANG YP, et al. A network pharmacology approach to investigate the blood enriching mechanism of Danggui buxue Decoction[J]. J Ethnopharmacol, 2019, 235:227-242. [7] ZHANG R, HAN X, HUANG T, et al. Danggui Buxue Tang inhibited mesangial cell proliferation and extracellular matrix accumulation through GAS5/NF-κB pathway[J]. Biosci Rep, 2019, 39(10): BSR20181740. doi: 10.1042/BSR20181740 [8] WANG WK, ZHOU Y, FAN L, et al. The antidepressant-like effects of Danggui Buxue Decoction in GK rats by activating CREB/BDNF/TrkB signaling pathway[J]. Phytomedicine, 2021, 89: 153600. doi: 10.1016/j.phymed.2021.153600 [9] GAO DH, GUO YJ, LI XJ, et al. An aqueous extract of Radix astragali, Angelica sinensis, and Panax notoginseng is effective in preventing diabetic retinopathy[J]. Evid Based Complement Alternat Med, 2013, 2013: 578165. [10] 薛梅, 卞勇, 周俊杰, 等. 当归补血汤主要吸收成分对GK大鼠肾保护作用研究[J]. 南京中医药大学学报, 2018, 34(2): 190-193. http://xb.njucm.edu.cn/article/id/ZR2018_0219XUE M, BIAN Y, ZHOU JJ, et al. Renal protective effect of absorbed bioactive compounds of Danggui buxue decoction on GK rats[J]. J Nanjing Univ Tradit Chin Med, 2018, 34(2): 190-193. http://xb.njucm.edu.cn/article/id/ZR2018_0219 [11] CALLE P, HOTTER G. Macrophage phenotype and fibrosis in diabetic nephropathy[J]. Int J Mol Sci, 2020, 21(8): 2806. doi: 10.3390/ijms21082806 [12] ZENG LF, XIAO Y, SUN L. A glimpse of the mechanisms related to renal fibrosis in diabetic nephropathy[J]. Adv Exp Med Biol, 2019, 1165: 49-79. [13] BROSIUS FC 3rd, ALPERS CE, BOTTINGER EP, et al. Mouse models of diabetic nephropathy[J]. J Am Soc Nephrol, 2009, 20(12), 2503-2512. doi: 10.1681/ASN.2009070721 [14] WANG JP, FANG CY, WANG SX, et al. Danggui Buxue Tang ameliorates bleomycin-induced pulmonary fibrosis in rats through inhibiting transforming growth factor-β1/Smad3/plasminogen activator inhibitor-1 signaling pathway[J]. J Tradit Chin Med, 2020, 40(2): 236-244. [15] CHEN Y, CHEN Q, LU J, et al. Effects of Danggui Buxue Decoction on lipid peroxidation and MMP-2/9 activities of fibrotic liver in rats[J]. Chin J Integr Med, 2009, 15(6): 435-441. doi: 10.1007/s11655-009-0435-y [16] WANG LN, MA JW, GUO CX, et al. Danggui Buxue Tang attenuates tubulointerstitial fibrosis via suppressing NLRP3 inflammasome in a rat model of unilateral ureteral obstruction[J]. Biomed Res Int, 2016, 2016: 9368483. [17] WANG JY, GAO YB, MA MF, et al. Effect of miR-21 on renal fibrosis by regulating MMP-9 and TIMP1 in kk-ay diabetic nephropathy mice[J]. Cell Biochem Biophys, 2013, 67(2): 537-546. doi: 10.1007/s12013-013-9539-2 [18] LIU HF, WANG XH, LIU SF, et al. Effects and mechanism of miR-23b on glucose-mediated epithelial-to-mesenchymal transition in diabetic nephropathy[J]. Int J Biochem Cell Biol, 2016, 70: 149-160. doi: 10.1016/j.biocel.2015.11.016 [19] WU LN, WANG QZ, GUO F, et al. microRNA-27a induces mesangial cell injury by targeting of PPARγ and its in vivo knockdown prevents progression of diabetic nephropathy[J]. Sci Rep, 2016, 6: 26072. doi: 10.1038/srep26072 [20] LAN HY. Diverse roles of TGF-β/Smads in renal fibrosis and inflammation[J]. Int J Biol Sci, 2011, 7(7): 1056-1067. doi: 10.7150/ijbs.7.1056 [21] 侯晓艳. microRNA-27a在糖尿病肾病肾小管间质纤维化中的作用及机制研究[D]. 广州: 南方医科大学, 2017.HOU XY. A study of the mechanism and effect of microRNA-27a on renal tubulointerstitial fibrosis in diabetic nephropathy[D]. Guangzhou: Southern Medical University, 2017. [22] KRUPA A, JENKINS R, LUO DD, et al. Loss of microRNA-192 promotes fibrogenesis in diabetic nephropathy[J]. J Am Soc Nephrol, 2010, 21(3): 438-447. doi: 10.1681/ASN.2009050530 [23] ZHENG ZJ, GUAN MP, JIA YJ, et al. The coordinated roles of miR-26a and miR-30c in regulating TGFβ1-induced epithelial-to-mesenchymal transition in diabetic nephropathy[J]. Sci Rep, 2016, 6: 37492. doi: 10.1038/srep37492 [24] SATO M, MURAGAKI Y, SAIKA S, et al. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction[J]. J Clin Invest, 2003, 112(10): 1486-1494. doi: 10.1172/JCI200319270 [25] KELLENBERGER T, KRAG S, DANIELSEN CC, et al. Differential effects of Smad3 targeting in a murine model of chronic kidney disease[J]. Physiol Rep, 2013, 1(7): e00181. doi: 10.1002/phy2.181 [26] ZHONG X, CHUNG ACK, CHEN HY, et al. Smad3-mediated upregulation of miR-21 promotes renal fibrosis[J]. J Am Soc Nephrol, 2011, 22(9): 1668-1681. doi: 10.1681/ASN.2010111168 -





 下载:
下载: