Effects of PAP-1 on Atherosclerosis and Cardioprotection in Rats
-
摘要:
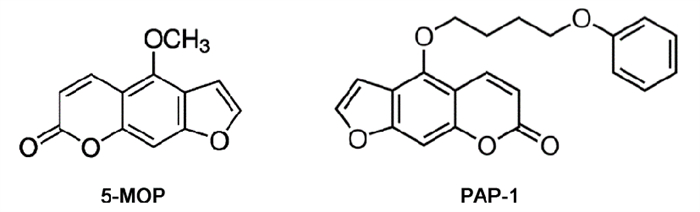
目的 探讨5-(4-苯氧基丁氧基)补骨脂素(PAP-1)对大鼠动脉粥样硬化(AS)的影响及心脏保护作用。 方法 建立AS大鼠模型,随机分为对照组、模型组、PAP-1组和阿托伐他汀(AV)阳性药组。HE染色观察颈动脉形态学改变,Western blot法检测巨噬细胞清道夫受体1(MSR1)和ATP结合盒转运体A1(ABCA1)蛋白表达,COD-PAP酶法检测血清总胆固醇(CHO)含量,ELISA法测定血清炎症因子TNF-α、IL-6和IL-1β含量,麦胚凝集素(WGA)染色测量心肌细胞面积,Vevo 3100高分辨动物超声影像系统观察各组大鼠左心室形态并计算心功能,Masson和免疫组织化学法检查心肌组织纤维化程度。 结果 PAP-1可以降低AS大鼠血清CHO含量和炎症因子TNF-α、IL-6和IL-1β的水平;下调AS大鼠颈主动脉MSR1,上调ABCA1蛋白表达;减轻AS大鼠颈主动脉管壁增厚。此外,PAP-1可以抑制AS大鼠心肌细胞面积增大和心室壁增厚,减轻心肌组织纤维化程度。 结论 PAP-1对AS大鼠具有一定保护作用,可能与降低血清血脂和炎症因子水平,调控MSR1和ABCA1蛋白表达有关。PAP-1还对AS大鼠心脏具有保护作用,表现为改善AS大鼠心脏结构和功能异常,抑制心肌纤维化等。 -
关键词:
- 5-(4-苯氧基丁氧基)补骨脂素 /
- 动脉粥样硬化 /
- 心脏保护
Abstract:OBJECTIVE To explore the effect of 5-(4-phenoxybutoxy) psoralen (PAP-1) on atherosclerosis (AS) and cardioprotection in rats. METHODS The AS model was established in rats, and the rats were randomly divided into control, model, PAP-1 and AV groups. HE staining was used to observe the carotid artery morphology. Western blot was performed to detect the expressions of MSR1 and ABCA1. Serum total cholesterol (CHO) was determined by COD-PAP enzymatic method. ELISA method was used to measure the levels of inflammatory cytokines TNF-α, IL-1β and IL-6. The cardiomyocyte areas were evaluated by WGA staining. Vevo 3100 doppler echocardiography was used to observe ventricular ultrasound images and calculate cardiac function. The levels of cardiomyocyte fibrosis and collagen deposition were detected by Masson and immunohistochemical staining. RESULTS PAP-1 intervention reduced the levels of serum CHO and inflammatory cytokines TNF-α, IL-1β and IL-6. PAP-1 reversed the protein expressions of MSR1 and ABCA1. PAP-1 inhibited cardiac enlargement and structural remodeling, and reduced cardiomyocyte fibrosis accumulation. CONCLUSION PAP-1 has a certain role in AS rats, which may be related to the regulation of the expression of MSR1 and ABCA1 and reduction of serum CHO. PAP-1 also has a cardioprotection effect on AS rats via alleviation of cardiac structure enlargement, function disorder and fibrosis. -
Key words:
- 5-(4-phenoxybutoxy) psoralen /
- atherosclerosis /
- cardioprotection
-
表 1 大鼠超声心动图参数(x±s,n=4)
组别 EF/% LVAWs/mm LVAWd/mm LVPWs/mm LVPWd/mm 对照组 78.81±0.93 3.17±0.15 1.97±0.11 3.45±0.20 2.29±0.23 模型组 73.56±5.89 3.90±0.13** 2.28±0.07* 3.51±0.08 2.42±0.06 PAP-1组 78.18±1.61 3.21±0.12## 2.15±0.01# 3.66±0.26 2.41±0.06 AV组 80.72±1.74 3.40±0.22# 2.20±0.14 3.37±0.39 2.32±0.25 注:与对照组比较,*P<0.05,**P<0.01;与模型组比较,#P<0.05,##P<0.01。 -
[1] WANG X, BAI M. CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro[J]. BMC Cardiovasc Disord, 2021, 21(1): 51. doi: 10.1186/s12872-020-01800-x [2] CHISTIAKOV DA, MELNICHENKO AA, MYASOEDOVA VA, et al. Mechanisms of foam cell formation in atherosclerosis[J]. J Mol Med, 2017, 95(11): 1153-1165. doi: 10.1007/s00109-017-1575-8 [3] MISHRA S, BEDJA D, AMUZIE C, et al. Prevention of cardiac hypertrophy by the use of a glycosphingolipid synthesis inhibitor in ApoE-/- mice[J]. Biochem Biophys Res Commun, 2015, 465(1): 159-164. doi: 10.1016/j.bbrc.2015.07.159 [4] QIN YW, YE P, HE JQ, et al. Simvastatin inhibited cardiac hypertrophy and fibrosis in apolipoprotein E-deficient mice fed a "Western-style diet" by increasing PPAR α and γ expression and reducing TC, MMP-9, and Cat S levels[J]. Acta Pharmacol Sin, 2010, 31(10): 1350-1358. doi: 10.1038/aps.2010.109 [5] VINCELETTE J, MARTIN-MCNULTY B, VERGONA R, et al. Reduced cardiac functional reserve in apolipoprotein E knockout mice[J]. Transl Res, 2006, 148(1): 30-36. doi: 10.1016/j.lab.2006.03.007 [6] 杜文婷, 顾耘. 新型调脂抗动脉粥样硬化药物进展概况[J]. 当代医学, 2020, 26(1): 192-194. doi: 10.3969/j.issn.1009-4393.2020.01.084 [7] 吴晓娟, 林小娟, 佟晓永. 抗动脉粥样硬化药物的研究进展[J]. 生命科学仪器, 2018, 16(6): 15-24. https://www.cnki.com.cn/Article/CJFDTOTAL-SBKY201806002.htm [8] BODENDIEK SB, MAHIEUX C, HANSEL W, et al. 4-Phenoxybutoxy-substituted heterocycles: A structure-activity relationship study of blockers of the lymphocyte potassium channel Kv1.3[J]. Eur J Med Chem, 2009, 44(5): 1838-1852. doi: 10.1016/j.ejmech.2008.10.033 [9] 丁燕, 傅友, 单兰兰, 等. 佛手柑内酯对双氧水诱导人脐静脉内皮细胞衰老的影响[J]. 中国组织工程研究, 2016, 20(46): 6885-6892. doi: 10.3969/j.issn.2095-4344.2016.46.006 [10] LORBEK G, LEWINSKA M, ROZMAN D. Cytochrome P450s in the synthesis of cholesterol and bile acids from mouse models to human diseases[J]. FEBS J, 2012, 279(9): 1516-1533. doi: 10.1111/j.1742-4658.2011.08432.x [11] 杨英来, 杨涛, 杨玉华, 等. 超高效液相色谱法测定浓缩当归丸和当归药材中光毒性化合物香豆素[J]. 分析化学, 2013, 41(11): 1744-1748. https://www.cnki.com.cn/Article/CJFDTOTAL-FXHX201311029.htm [12] ZAJDELA F, BISAGNI E. 5-Methoxypsoralen, the melanogenic additive in sun-tan preparations, is tumorigenic in mice exposed to 365 nm u. v. radiation[J]. Carcinogenesis, 1981, 2(2): 121-127. doi: 10.1093/carcin/2.2.121 [13] SCHMITZ A, SANKARANARAYANAN A, AZAM P, et al. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases[J]. Mol Pharmacol, 2005, 68(5): 1254-1270. doi: 10.1124/mol.105.015669 [14] HAO B, CHEN ZW, ZHOU XJ, et al. Identification of phase-Ⅰ metabolites and chronic toxicity study of the Kv1.3 blocker PAP-1 (5-(4-phenoxybutoxy)psoralen) in the rat[J]. Xenobiotica, 2011, 41(3): 198-211. doi: 10.3109/00498254.2010.532886 [15] BEETON C, WULFF H, STANDIFER NE, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases[J]. Proc Natl Acad Sci USA, 2006, 103(46): 17414-17419. doi: 10.1073/pnas.0605136103 [16] PEREIRA LE, VILLINGER F, WULFF H, et al. Pharmacokinetics, toxicity, and functional studies of the selective Kv1.3 channel blocker 5-(4-phenoxybutoxy)psoralen in rhesus macaques[J]. Exp Biol Med, 2007, 232(10): 1338-1354. doi: 10.3181/0705-RM-148 [17] ZHOU QL, WANG TY, LI M, et al. Alleviating airway inflammation by inhibiting ERK-NF-κB signaling pathway by blocking Kv1.3 channels[J]. Int Immunopharmacol, 2018, 63: 110-118. doi: 10.1016/j.intimp.2018.07.009 [18] MEI Y, FANG C, DING S, et al. PAP-1 ameliorates DSS-induced colitis with involvement of NLRP3 inflammasome pathway[J]. Int Immunopharmacol, 2019, 75: 105776. doi: 10.1016/j.intimp.2019.105776 [19] WU X, XU R, CAO M, et al. Effect of the Kv1.3 voltage-gated potassium channel blocker PAP-1 on the initiation and progress of atherosclerosis in a rat model[J]. Heart Vessels, 2015, 30(1): 108-114. doi: 10.1007/s00380-013-0462-7 [20] FONSECA FA, PAIVA TB, SILVA EG, et al. Dietary magnesium improves endothelial dependent relaxation of balloon injured arteries in rats[J]. Atherosclerosis, 1998, 139(2): 237-242. doi: 10.1016/S0021-9150(98)00069-0 [21] SELATHURAI A, DESWAERTE V, KANELLAKIS P, et al. Natural killer (NK) cells augment atherosclerosis by cytotoxic-dependent mechanisms[J]. Cardiovasc Res, 2014, 102(1): 128-137. doi: 10.1093/cvr/cvu016 [22] AZAM P, SANKARANARAYANAN A, HOMERICK D, et al. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis[J]. J Invest Dermatol, 2007, 127(6): 1419-1429. doi: 10.1038/sj.jid.5700717 [23] UNIS A, ABDELBARY A, HAMZA M. Comparison of the effects of escitalopram and atorvastatin on diet-induced atherosclerosis in rats[J]. Can J Physiol Pharmacol, 2014, 92(3): 226-233. doi: 10.1139/cjpp-2013-0168 [24] LI T, YAO W. Therapeutic effect of irbesartan combined with atorvastatin calcium in the treatment of rats with coronary heart disease[J]. Exp Ther Med, 2018, 16(5): 4119-4123. http://www.onacademic.com/detail/journal_1000040884362310_1132.html [25] 聂忠富, 孙小燕, 张太平, 等. 人参皂苷Compound K对动脉粥样硬化大鼠氧化应激, 炎症因子和血管活性物质的影响[J]. 河北中医, 2019, 41(7): 1042-1047. doi: 10.3969/j.issn.1002-2619.2019.07.019 [26] ZHANG N, ZHANG M, LIU RT, et al. Statins reduce the expressions of Tim-3 on NK cells and NKT cells in atherosclerosis[J]. Eur J Pharmacol, 2018, 821: 49-56. doi: 10.1016/j.ejphar.2017.12.050 [27] YU XH, FU YC, ZHANG DW, et al. Foam cells in atherosclerosis[J]. Clin Chim Acta, 2013, 424: 245-252. doi: 10.1016/j.cca.2013.06.006 [28] 郑双, 谭伟江, 李想, 等. 紫苏籽提取物在ApoE-/-小鼠中的抗动脉粥样硬化和心脏保护作用[J]. 中国实验动物学报, 2019, 27(6): 683-691. https://www.cnki.com.cn/Article/CJFDTOTAL-ZGSD201906023.htm [29] 杨舒涵, 王博, 孙美莎, 等. 多肽化合物urantide抑制动脉粥样硬化大鼠心脏Ⅰ型胶原的表达[J]. 解剖学报, 2020, 51(1): 103-108. https://www.cnki.com.cn/Article/CJFDTOTAL-JPXB202001023.htm [30] 张维忠, 赵清. 心肌纤维化时胶原组成变化的实验研究[J]. 上海第二医科大学学报, 1997, 17(5): 347-349. https://www.cnki.com.cn/Article/CJFDTOTAL-SHEY199705011.htm [31] 郭志坤, 周俐红, 李和. 大鼠心脏缺血再灌注对Ⅰ和Ⅲ型胶原纤维的影响[J]. 解剖学杂志, 2004, 27(4): 363-365. https://www.cnki.com.cn/Article/CJFDTOTAL-JPXZ200404010.htm -





 下载:
下载: