Ligustilide Inhibits RANKL-Induced Osteoclast Differentiation in RAW264.7 Cells and Its Mechanism Related by GPER
-
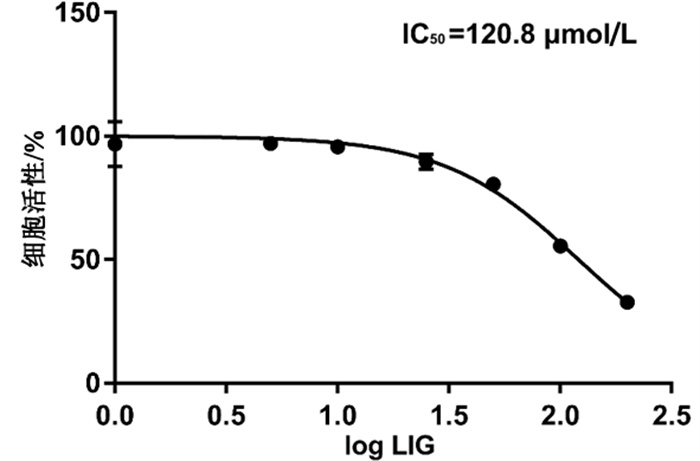
摘要: 目的 观察藁本内酯(LIG)对核因子κB受体活化因子配体(RANKL)诱导RAW264.7向破骨细胞分化的影响,并探讨该作用与G蛋白偶联雌激素受体(GPER)的相关机制。方法 体外培养RAW264.7细胞,RANKL诱导破骨细胞分化,并用LIG进行干预。通过抗酒石酸酸性磷酸酶(TRAP)活性检测和TRAP染色法评价破骨细胞形成和分化能力;qPCR法检测GPER及破骨细胞相关基因mRNA水平;Western blot法检测GPER蛋白表达;采用GPER特异性拮抗剂G36进行干预,观察LIG干预破骨细胞分化的作用变化。结果 LIG浓度为10 μmol/L时,TRAP活性检测结果显示,TRAP活性显著降低(P < 0.01);TRAP染色结果显示,与RANKL组相比,LIG组TRAP阳性细胞形成减少(P < 0.001);qPCR检测结果显示,与RANKL组相比,LIG组树突状细胞-特异性跨膜蛋白(DC-STAMP)、活化T细胞核因子(NFATc1)、组织蛋白酶K(CTSK)和核因子κB受体(RANK)的mRNA水平显著降低(P < 0.05, P < 0.001),而GPER mRNA表达明显升高(P < 0.001);Western blot结果显示,与RANKL组相比,LIG组GPER蛋白表达升高(P < 0.001)。与LIG组比较,LIG+G36组TRAP阳性细胞数升高(P < 0.01),TRAP活性增强(P < 0.05),DC-STAMP、NFATc1、CTSK、RANK mRNA的表达升高(P < 0.05)。结论 LIG能够抑制RANKL诱导RAW264.7向破骨细胞分化,其机制可能是促进GPER表达,减少RANK和下游转录因子NFATc1的表达,抑制破骨细胞分化和骨吸收功能。
-
关键词:
- 骨质疏松 /
- 藁本内酯 /
- 破骨细胞 /
- G蛋白偶联雌激素受体
Abstract: OBJECTIVE To observe the effect of ligustilide (LIG) on the differentiation of RAW264.7 cells into osteoclasts induced by receptor activator of nuclear factor-κB ligand (RANKL), and to explore the mechanism from the perspective of G protein coupled estrogen receptor (GPER).METHODS RAW264.7 cells were cultured in vitro and osteoclast-like cells differentiation were induced by RANKL. Osteoclast formation and differentiation were identified by tartrate resistant acid phosphatase (TRAP) staining and TRAP enzyme activity. Expressions of GPER and osteoclast differentiation related marker genes were observed by qPCR. GPER protein was assessed by Western blot. The GPER specific antagonist G36 was utilized to detect the effect of LIG on osteoclast differentiation through TRAP staining, TRAP enzyme activity, qPCR and phalloidin staining.RESULTS TRAP enzyme activity of LIG (10 μmol/L) group was much lower than that of RANKL group (P < 0.01). TRAP staining showed that the number of TRAP positive cells of LIG group was smaller than that of RANKL group (P < 0.001). The qPCR results showed that the expressions of DC-STAMP, NFATc1, CTSK and RANK mRNA of LIG group were significantly lower than those of RANKL group (P < 0.05, P < 0.001), and the expression of GPER mRNA was significantly up-regulated (P < 0.001). Western blot showed that the expression of GPER protein of LIG group was higher than that of RANKL group (P < 0.001). After the treatment with G36, the effect of LIG on osteoclast-like cells differentiation were attenuated, the number of TRAP positive cells increased (P < 0.01), TRAP enzyme activity was enhanced (P < 0.05) and the mRNA expressions of DC-STAMP, NFATc1, CTSK and RANK significantly increased (P < 0.05).CONCLUSION LIG can promote the expression of GPER, reduce the expression of RANK and downstream transcription factor NFATc1 in RANKL induced osteoclast, which may be one of the mechanisms involved in LIG suppressing osteoclast differentiation and bone resorption.-
Key words:
- osteoporosis /
- ligustilide /
- osteoclast /
- G protein coupled estrogen receptor
-
表 1 引物序列
引物 序列(5'→3') CTSK CAGTAGCCACGCTTCCTATCC(Forward) ACTGGGTGTCCAGCATTTCC(Reverse) NFATc1 CCCGTCACATTCTGGTCCAT(Forward) CAAGTAACCGTGTAGCTCCACAA(Reverse) DC-STAMP CTCGCCGGGCTTCTGCTCAT(Forward) CCGCTGTTGGTGCCTCTCCT(Reverse) RANK CCAGGACAGGGCTGATGAGAA(Forward) TGGCTGACATACACCACGATGA(Reverse) GPER TCCTCATCCTGGTGGTGAAC(Forward) GTCGTAGTACTGCTCGTCCA(Reverse) GAPDH GGTTGTCTCCTGCGACTTCA(Forward) TGGTCCAGGGTTTCTTACTCC(Reverse) -
[1] 中华医学会骨质疏松和骨矿盐疾病分会. 中国骨质疏松症流行病学调查及"健康骨骼"专项行动结果发布[J]. 中华骨质疏松和骨矿盐疾病杂志, 2019, 12(4): 317-318. doi: 10.3969/j.issn.1674-2591.2019.04.001 [2] CUMMINGS SR, SAN MARTIN J, MCCLUNG MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis[J]. N Engl J Med, 2009, 361(8): 756-765. doi: 10.1056/NEJMoa0809493 [3] OTTO S, PAUTKE C, VAN DEN WYNGAERT T, et al. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases[J]. Cancer Treat Rev, 2018, 69: 177-187. doi: 10.1016/j.ctrv.2018.06.007 [4] 中华中医药学会. 绝经后骨质疏松症(骨痿)中医药诊疗指南(2019年版)[J]. 中医正骨, 2020, 32(2): 1-13. doi: 10.3969/j.issn.1001-6015.2020.02.001 [5] 赖满香, 林基伟, 廖利平, 等. 基于中医传承辅助系统的治疗原发性骨质疏松症方剂组方规律分析[J]. 中国实验方剂学杂志, 2017, 23(9): 202-207. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX201709036.htm [6] 唐文文, 李国琴, 晋小军. 不同生长年限当归挥发油对比研究[J]. 中国实验方剂学杂志, 2013, 19(19): 163-166. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX201319048.htm [7] YANG F, LIN ZW, HUANG TY, et al. Ligustilide, a major bioactive component of Angelica sinensis, promotes bone formation via the GPR30/EGFR pathway[J]. Sci Rep, 2019, 9(1): 6991. doi: 10.1038/s41598-019-43518-7 [8] RIGGS BL, KHOSLA S, MELTON LJ. A unitary model for involutional osteoporosis: Estrogen deficiency causes both type Ⅰ and type Ⅱ osteoporosis in postmenopausal women and contributes to bone loss in aging men[J]. J Bone Miner Res, 1998, 13(5): 763-773. doi: 10.1359/jbmr.1998.13.5.763 [9] CAULEY JA, ROBBINS J, CHEN Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: The women's health initiative randomized trial[J]. JAMA, 2003, 290(13): 1729-1738. doi: 10.1001/jama.290.13.1729 [10] Collaborative Group on Epidemiological Studies of Ovarian Cancer. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies[J]. Lancet, 2015, 385(9980): 1835-1842. doi: 10.1016/S0140-6736(14)61687-1 [11] LUO J, LIU DM. Does GPER really function as a G protein-coupled estrogen receptor in vivo?[J]. Front Endocrinol, 2020, 11: 148. doi: 10.3389/fendo.2020.00148 [12] REVANKAR CM, CIMINO DF, SKLAR LA, et al. A transmembrane intracellular estrogen receptor mediates rapid cell signaling[J]. Science, 2005, 307(5715): 1625-1630. doi: 10.1126/science.1106943 [13] THOMAS P, PANG Y, FILARDO EJ, et al. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells[J]. Endocrinology, 2005, 146(2): 624-632. doi: 10.1210/en.2004-1064 [14] HEINO TJ, CHAGIN AS, SÄVENDAHL L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone[J]. J Endocrinol, 2008, 197(2): R1-R6. doi: 10.1677/JOE-07-0629 [15] LI J, XIANG L, JIANG XT, et al. Investigation of bioeffects of G protein-coupled receptor 1 on bone turnover in male mice[J]. J Orthop Translat, 2017, 10: 42-51. doi: 10.1016/j.jot.2017.05.001 [16] MARTENSSON UE, SALEHI SA, WINDAHL S, et al. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice[J]. Endocrinology, 2009, 150(2): 687-698. doi: 10.1210/en.2008-0623 [17] MASUHARA M, TSUKAHARA T, TOMITA K, et al. A relation between osteoclastogenesis inhibition and membrane-type estrogen receptor GPR30[J]. Biochem Biophys Rep, 2016, 8: 389-394. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5614543/pdf/main.pdf [18] KANG WB, DENG YT, WANG DS, et al. Osteoprotective effects of estrogen membrane receptor GPR30 in ovariectomized rats[J]. J Steroid Biochem Mol Biol, 2015, 154: 237-244. doi: 10.1016/j.jsbmb.2015.07.002 [19] WANG DR, LI J, FENG W, et al. Ligustilide suppresses RANKL-induced osteoclastogenesis and bone resorption via inhibition of RANK expression[J]. J Cell Biochem, 2019, 120(11): 18667-18677. doi: 10.1002/jcb.29153 [20] PARK JH, LEE NK, LEE SY. Current understanding of RANK signaling in osteoclast differentiation and maturation[J]. Mol Cells, 2017, 40(10): 706-713. http://www.molcells.org/file/contents_40_10.pdf [21] DESMAWATI D, SULASTRI D. Phytoestrogens and their health effect[J]. Open Access Maced J Med Sci, 2019, 7(3): 495-499. doi: 10.3889/oamjms.2019.086 [22] ROWE IJ, BABER RJ. The effects of phytoestrogens on postmenopausal health[J]. Climacteric, 2021, 24(1): 57-63. doi: 10.1080/13697137.2020.1863356 [23] QIAO C, YE WJ, LI S, et al. Icariin modulates mitochondrial function and apoptosis in high glucose-induced glomerular podocytes through G protein-coupled estrogen receptors[J]. Mol Cell Endocrinol, 2018, 473: 146-155. doi: 10.1016/j.mce.2018.01.014 -





 下载:
下载: