Preparation and in vivo and in vitro Study of Piperine-Theanine Co-Amorphous Complex
-
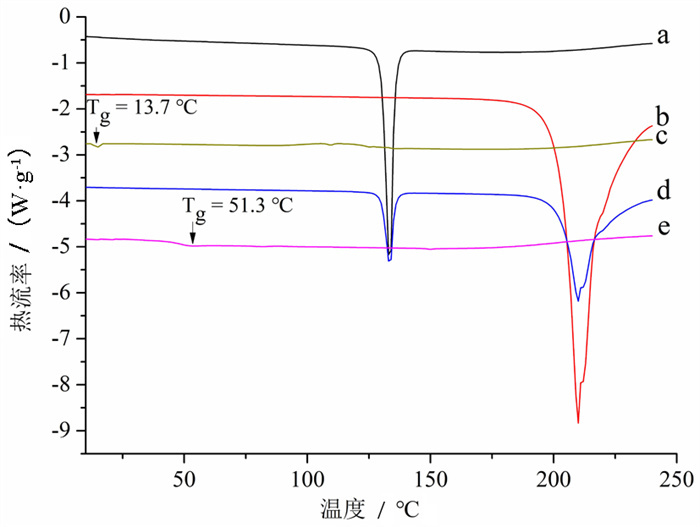
摘要: 目的 将胡椒碱(PIP)和茶氨酸(THE)联合制备成胡椒碱-茶氨酸共无定型复合物(PIP-THE CAC),提高PIP的溶出度以及生物利用度。方法 通过淬火冷却法制备PIP-THE CAC,采用差示扫描量热分析(DSC)、粉末X-射线衍射(XRPD)、傅里叶红外光谱(FTIR)和扫描电镜(SEM)对制备的PIP-THE CAC进行表征分析,对PIP-THE CAC在漏槽和非漏槽条件下的体外溶出进行评价,并考察PIP-THE CAC的物理稳定性。此外,在大鼠体内进行PIP-THE CAC的药代动力学研究。结果 DSC和XRPD结果表明成功制备PIP-THE CAC。FTIR证实在PIP-THE CAC中,PIP与THE之间发生了分子间氢键相互作用,导致PIP-THE CAC具有良好的物理稳定性。SEM观察发现PIP-THE CAC呈不规则块状、颗粒状,已不见PIP和THE的特征。体外溶出实验表明,与PIP原料药及PIP-THEPM相比,PIP-THE CAC具有更高的溶出速率和溶出度并且可维持长时间的超饱和程度。药代动力学实验结果表明,与PIP原料药组比较,PIP-THE CAC组Cmax、tmax、AUC0-24 h、AUC0-∞显著增加(P < 0.01),PIP的Cmax和生物利用度分别提高了2.03、1.93倍(P < 0.01)。结论 将PIP和THE联合制备成的PIP-THE CAC能有效地改善PIP的溶解度、体外溶出度以及生物利用度。Abstract: OBJECTIVE To prepare piperine-theanine co-amorphous complex (PIP-THE CAC) and improve dissolution and bioavailability of PIP.METHODS The PIP-THE CAC was prepared by quench cooling, characterized by DSC, XRPD, FTIR, SEM and evaluated by in vitro dissolution under sink and non-sink conditions. The physical stability of PIP-THE CAC was also investigated. Besides, the bioavailability study of PIP-THE CAC was conducted by pharmacokinetic tests in rats.RESULTS DSC and XRPD indicated that PIP-THE CAC was successfully prepared. FTIR confirmed that there was intermolecular hydrogen bond interaction between PIP and THE in the prepared CAC, resulting in excellent physical stability. SEM showed that the CAC was irregular, lumpy and granular, and the characteristics of PIP and THE were disappeared. Besides, compared with pure PIP and PIP-THE physical mixture, PIP-THE CAC possessed a higher dissolution rate and a higher dissolution degree, and maintained supersaturated degree for long time in vitro dissolution experiments. Pharmacokinetic results showed that Cmax, tmax, AUC0-24 h and AUC0-∞ of PIP-THE CAC group increased significantly (P < 0.01) compared with raw PIP, and the Cmax and bioavailability of PIP increased by 2.03 and 1.93 times, respectively (P < 0.01).CONCLUSION PIP-THE CAC prepared by THE co-amorphization with PIP can effectively improve the solubility/dissolution and oral bioavailability of PIP.
-
Key words:
- piperine /
- theanine /
- co-amorphous complex /
- in vitro dissolution /
- stability /
- bioavailability
-
表 1 PIP原料药组、PIP-THE PM组和PIP-THE CAC组中PIP的药代动力学参数(x±s, n=6)
药代动力学参数 PIP PIP-THE PM PIP-THE CAC Cmax(μg·mL-1) 9.67±2.37 8.89±0.50 19.65±1.13**## tmax(h) 3.83±1.17 3.92±1.84 2.33±0.52*# t1/2(h) 6.22±2.67 5.50±1.37 6.27±2.12 AUC0-24 h(μg·h·mL-1) 105.40±33.37 101.92±20.91 203.25±15.96**## AUC0-∞(μg·h·mL-1) 119.50±54.44 112.54±17.18 224.30±28.61**## 注:与PIP原料药比较,*P < 0.05,**P < 0.01;与PIP-THE PM组比较,#P < 0.05,##P < 0.01。 -
[1] MA ZG, YUAN YP, ZHANG X, et al. Piperine attenuates pathological cardiac fibrosis via PPAR-γ/AKT Pathways[J]. EbioMedicine, 2017, 18: 179-187. doi: 10.1016/j.ebiom.2017.03.021 [2] 白音夫, 杨宏昕. 荜茇挥发油对动物实验性胃溃疡的保护作用[J]. 中草药, 2000, 31(1): 40-41. https://www.cnki.com.cn/Article/CJFDTOTAL-ZCYO200001021.htm [3] LEE SA, HONG SS, HAN XH, et al. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity[J]. Chem Pharm Bull, 2005, 53(7): 832-835. doi: 10.1248/cpb.53.832 [4] SUNILA ES, KUTTAN G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine[J]. J Ethnopharmacol, 2004, 90(2/3): 339-346. http://www.onacademic.com/detail/journal_1000035405045810_f399.html [5] ONONIWU IM, IBENEME CE, EBONG OO. Effects of piperine on gastric acid secretion in albino rats[J]. Afr J Med Med Sci, 2002, 31(4): 293-295. http://europepmc.org/abstract/MED/15027765 [6] KHAJURIA A, THUSU N, ZUTSHI U, et al. Piperine modulation of carcinogen induced oxidative stress in intestinal mucosa[J]. Mol Cell Biochem, 1998, 189(1/2): 113-118. doi: 10.1023/A:1006877614411 [7] DOGRA RKS, KHANNA S, SHANKER R. Immunotoxicological effects of piperine in mice[J]. Toxicology, 2004, 196(3): 229-236. doi: 10.1016/j.tox.2003.10.006 [8] 吴珍菊, 夏学进, 黄雪松. 胡椒碱平衡溶解度和表观油水分配系数的测定[J]. 暨南大学学报(自然科学与医学版), 2012, 33(5): 473-476. doi: 10.3969/j.issn.1000-9965.2012.05.008 [9] 余丹妮, 伍薇, 祁玮玮, 等. 女贞子三萜-胡椒碱共无定型复合物的表征和体外溶出度评价[J]. 中草药, 2018, 49(3): 561-568. https://www.cnki.com.cn/Article/CJFDTOTAL-ZCYO201803009.htm [10] HSU CM, YU SC, TSAI FJ, et al. Characterization of in vitro and in vivo bioactivity of a ferulic acid-2-Hydroxypropyl-β-cyclodextrin inclusion complex[J]. Colloids Surf B Biointerfaces, 2019, 180: 68-74. doi: 10.1016/j.colsurfb.2019.04.020 [11] AHMED S, CORVIS Y, GAHOUAL R, et al. Conception of nanosized hybrid liposome/poloxamer particles to thicken the interior core of liposomes and delay hydrophilic drug delivery[J]. Int J Pharm, 2019, 567: 118488. doi: 10.1016/j.ijpharm.2019.118488 [12] VASCONCELOS T, MARQUES S, DAS NEVES J, et al. Amorphous solid dispersions: Rational selection of a manufacturing process[J]. Adv Drug Deliv Rev, 2016, 100: 85-101. doi: 10.1016/j.addr.2016.01.012 [13] ABDULKARIM M, SHARMA PK, GUMBLETON M. Self-emulsifying drug delivery system: Mucus permeation and innovative quantification technologies[J]. Adv Drug Deliv Rev, 2019, 142: 62-74. doi: 10.1016/j.addr.2019.04.001 [14] HOU GZ, WANG ZY, MA HG, et al. High-temperature stable plasmonic and cavity resonances in metal nanoparticle-decorated silicon nanopillars for strong broadband absorption in photothermal applications[J]. Nanoscale, 2019, 11(31): 14777-14784. doi: 10.1039/C9NR05019A [15] TRUBITSYN G, NGUYEN VN, DI TOMMASO C, et al. Impact of covalently Nile Red and covalently Rhodamine labeled fluorescent polymer micelles for the improved imaging of the respective drug delivery system[J]. Eur J Pharm Biopharm, 2019, 142: 480-487. doi: 10.1016/j.ejpb.2019.07.020 [16] SHI XJ, SONG SJ, DING ZJ, et al. Improving the solubility, dissolution, and bioavailability of ibrutinib by preparing it in a coamorphous state with saccharin[J]. J Pharm Sci, 2019, 108(9): 3020-3028. doi: 10.1016/j.xphs.2019.04.031 [17] FUNG MH, DEVAULT M, KUWATA KT, et al. Drug-excipient interactions: Effect on molecular mobility and physical stability of ketoconazole-organic acid coamorphous systems[J]. Mol Pharm, 2018, 15(3): 1052-1061. doi: 10.1021/acs.molpharmaceut.7b00932 [18] CHAVAN RB, THIPPARABOINA R, KUMAR D, et al. Co amorphous systems: A product development perspective[J]. Int J Pharm, 2016, 515(1/2): 403-415. http://www.researchgate.net/profile/Rahul_Chavan13/publication/309292884_Co_Amorphous_Systems_A_Product_Development_Perspective/links/580afcca08aeef1bfee3fb42.pdf [19] OJARINTA R, HEIKKINEN AT, SIEVANEN E, et al. Dissolution behavior of co-amorphous amino acid-indomethacin mixtures: The ability of amino acids to stabilize the supersaturated state of indomethacin[J]. Eur J Pharm Biopharm, 2017, 112: 85-95. doi: 10.1016/j.ejpb.2016.11.023 [20] LENZ E, JENSEN KT, BLAABJERG LI, et al. Solid-state properties and dissolution behaviour of tablets containing co-amorphous indomethacin-arginine[J]. Eur J Pharm Biopharm, 2015, 96: 44-52. doi: 10.1016/j.ejpb.2015.07.011 [21] LENZ E, LOBMANN K, RADES T, et al. Hot melt extrusion and spray drying of co-amorphous indomethacin-arginine with polymers[J]. J Pharm Sci, 2017, 106(1): 302-312. doi: 10.1016/j.xphs.2016.09.027 [22] PETRY I, LOBMANN K, GROHGANZ H, et al. Undesired co-amorphisation of indomethacin and arginine during combined storage at high humidity conditions[J]. Int J Pharm, 2018, 544(1): 172-180. doi: 10.1016/j.ijpharm.2018.04.026 [23] HENG WL, SU ML, CHENG H, et al. Incorporation of complexation into a coamorphous system dramatically enhances dissolution and eliminates gelation of amorphous lurasidone hydrochloride[J]. Mol Pharm, 2020, 17(1): 84-97. doi: 10.1021/acs.molpharmaceut.9b00772 [24] WU W, LӦBMANN K, SCHNITZKEWITZ J, et al. Dipeptides as co-formers in co-amorphous systems[J]. Eur J Pharm Biopharm, 2019, 134: 68-76. doi: 10.1016/j.ejpb.2018.11.016 [25] HUANG R, HAN J, WANG R, et al. Surfactant-free solid dispersion of BCS class Ⅳ drug in an amorphous chitosan oligosaccharide matrix for concomitant dissolution in vitro-permeability increase[J]. Eur J Pharm Sci, 2019, 130: 147-155. doi: 10.1016/j.ejps.2019.01.031 [26] WANG RN, HAN JW, JIANG A, et al. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine[J]. Int J Pharm, 2019, 561: 9-18. doi: 10.1016/j.ijpharm.2019.02.027 [27] 黄容, 陆昕怡, 韩加伟, 等. 姜黄素-胡椒碱固体分散体的制备与生物利用度研究[J]. 中草药, 2018, 49(19): 4528-4534. doi: 10.7501/j.issn.0253-2670.2018.19.010 [28] LOBMANN K, LAITINEN R, GROHGANZ H, et al. Coamorphous drug systems: Enhanced physical stability and dissolution rate of indomethacin and naproxen[J]. Mol Pharmaceutics, 2011, 8(5): 1919-1928. doi: 10.1021/mp2002973 [29] BEVERNAGE J, BROUWERS J, BREWSTER ME, et al. Evaluation of gastrointestinal drug supersaturation and precipitation: Strategies and issues[J]. Int J Pharm, 2013, 453(1): 25-35. doi: 10.1016/j.ijpharm.2012.11.026 -





 下载:
下载: