Advance in Research on Platycodonis Radix and Preliminary Analysis of Its Quality Marker Prediction
-
摘要: 桔梗是桔梗科植物桔梗Platycodon grandiflorum (Jacq.) A. DC的干燥根,主要含有皂苷、黄酮、多糖、甾醇和脂肪酸等化学成分,现代研究表明其具有祛痰止咳、抗炎、抗肿瘤、降血糖、抗肥胖、免疫调节等药理作用。从桔梗化学成分、药理作用、体内过程、毒性及溶血性和质量标准等5个方面进行了系统综述,并对其质量标志物进行了探讨,以期为桔梗的进一步研究与开发提供参考。Abstract: Platycodonis Radix is the dried root of Platycodon grandiflorum (Jacq.) A. DC, which mainly contains saponins, flavonoids, polysaccharides, sterols and fatty acids. Modern studies have shown that Platycodonis Radix has pharmacological effects such as expectant and cough relieving, anti-inflammatory, anti-tumor, hypoglycemic, anti-obesity and immunoregulation.In this paper, the chemical constituents, pharmacological activities, in vivo process, toxicity, hemolysis and quality standard of Platycodonis Radix were systematically reviewed, and the quality markers of Platycodonis Radix were also discussed, in order to provide reference for further research and development of Platycodonis Radix. KEYWORDS: Platycodonis Radix; chemical composition; pharmacological activities; Q-marker
-
Key words:
- Platycodonis Radix /
- chemical composition /
- pharmacological activities /
- Q-marker
-
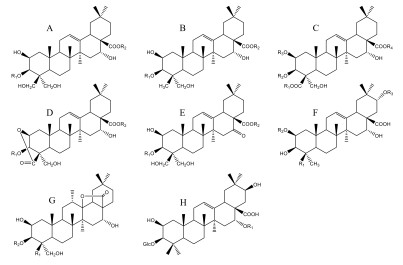
表 1 桔梗皂苷类化合物
No 化合物 分子式 类型 R1 R2 R3 R4 参考文献 1 Platycodigenin C30H48O7 A H H - - [3] 2 Platycodin A(2"-O-Acetyl platycodin D) C59H94O29 A Glc S8 - - [3] 3 Platycodin C(3"-O-Acetyl platycodin D) C59H94O29 A Glc S7 - - [3] 4 Platycodin D C57H92O28 A Glc S4 - - [4] 5 Platycodin D2 C63H102O33 A GEN S4 - - [5] 6 Platycodin D3 C63H102O33 A GEN S4 - - [4] 7 Platycodin J C57H90O29 A GlcA S4 - - [6] 8 Platycodin K C59H92O30 A GlcA S8 - - [6] 9 Platycodin L C59H92O30 A GlcA S7 - - [6] 10 2''-O-Acetyl platycodin D2 C65H104O34 A LAM S8 - - [7] 11 2''-O-Acetyl platycodin D3 C65H104O34 A GEN S8 - - [7] 12 3''-O-Acetyl platycodin D2 C65H104O34 A LAM S7 - - [8] 13 3''-O-Acetyl platycodin D3 C65H104O34 A GEN S7 - - [7] 14 Deapi-Platycodin D C52H84O24 A Glc S3 - - [4] 15 Deapi-Platycodin D2 C58H94O29 A LAM S3 - - [9] 16 Deapi-Platycodin D3 C58H94O29 A GEN S3 - - [4] 17 Deapi-3''-O-acetyl platycodin D C54H86O25 A Glc S5 - - [8] 18 Deapi-2''-O-acetyl platycodin D2 C60H96O30 A LAM S6 - - [7] 19 Platycoside A C58H94O29 A LAM S3 - - [10] 20 Platycoside B C54H86O25 A Glc S6 - - [10] 21 Platycoside C C54H86O25 A Glc S5 - - [10] 22 Platycoside E C69H112O38 A S1 S4 - - [11] 23 Platycoside F C47H76O20 A Glc S2 - - [12] 24 Platycoside G1(Deapi-platycoside E) C64H104O34 A S1 S3 - - [13] 25 Platycoside G2 C59H96O30 A GEN S2 - - [13] 26 Platycoside I C64H104O33 A S1 S3 - - [12] 27 Platycoside J C52H84O23 A Glc S3 - - [12] 28 Platycoside K C42H68O17 A LAM H - - [12] 29 Platycoside L C42H68O17 A GEN H - - [12] 30 Platycoside P C53H86O25 A LAM S2 - - [14] 31 β-Gentiotriosylplatycodigenin C48H78O22 A S1 Ara - - [7] 32 3-O-β-D-Glucopyranosyl platycodigenin C36H58O12 A Glc H - - [15] 33 3-O-β-D-Glucopyranosyl platycodigenin methyl ester C37H60O12 A Glc CH3 - - [16] 34 3-O-β-Gentiotriosyl platycodigenin methyl ester C43H70O17 A GEN CH3 - - [16] 35 3-O-β-Laminaribiosyl platycodigenin methyl ester C43H70O17 A LAM CH3 - - [16] 36 Platycoside D C69H112O37 B S1 S4 - - [11] 37 Platycoside G3(Polygalacin D3) C63H102O32 B GEN S4 - - [13] 38 Platycoside H C58H94O28 B GEN S3 - - [12] 39 Platycoside N C53H86O24 B GEN S2 - - [17] 40 Polygalacic acid C30H48O6 B H H - - [3] 41 Polygalacin D C57H92O27 B Glc S4 - - [4] 42 Polygalacin D2 C63H102O32 B LAM S4 - - [7] 43 2''-O-Acetyl-polygalacin D C59H94O28 B Glc S8 - - [4] 44 2''-O-Acetyl-polygalacin D2 C65H104O33 B LAM S8 - - [9] 45 3''-O-Acetyl-polygalacin D C59H94O28 B Glc S7 - - [4] 46 3''-O-Acetyl-polygalacin D2 C65H104O33 B LAM S7 - - [9] 47 3''-O-Acetyl-polygalacin D3 C65H104O34 B GEN S7 - - [8] 48 Deapi-polygalacin D2 C58H94O28 B LAM S3 - - [8] 49 Deapi-polygalacin D3 C58H94O28 B GEN S3 - - [7] 50 Deapi-2''-O-acetyl polygalacin D2 C60H95O30 B LAM S6 - - [8] 51 Deapi-2''-O-acetyl polygalacin D3 C60H95O30 B GEN S6 - - [8] 52 Dexyl-2''-O-acetyl-polygalacin D3 C55H87O25 B GEN S9 - - [8] 53 β-Gentiobiosyl-platycodigenin C42H68O16 B GEN Ara - - [7] 54 3-O-β-D-glucopyranosyl polygalacic acid C36H58O11 B Glc H - - [18] 55 3-O-β-D-Laminaribiosyl polygalacic acid C42H68O16 B LAM H - - [18] 56 Methyl-3-O-β-D-glucopyranosyl polygalacate C37H60O11 B Glc CH3 - - [16] 57 Methyl-3-O-β-laminaribiosyl polygalacate C43H70O16 B LAM CH3 - - [16] 58 Platycogenic acid A C30H46O8 C H H H H [3] 59 Platyconic acid A C57H90O29 C H Glc H S4 [5] 60 Platyconic acid B C59H92O30 C H Glc H S7 [6] 61 Platyconic acid C C52H82O25 C H Glc H S3 [6] 62 Platyconic acid D C54H84O26 C H Glc H S8 [6] 63 Platyconic acid E C58H92O30 C H GEN H S3 [6] 64 Platycoside O C53H84O25 C CH3 Glc H S3 [19] 65 Platyconic acid A methyl ester C58H92O29 C CH3 Glc H S4 [5] 66 Methyl Platyconate A C58H92O29 C CH3 Glc H S4 [20] 67 Methyl 2-O-methyl platyconate A C59H94O29 C CH3 Glc CH3 S4 [20] 68 Dimethyl 2-O-methyl-3-O-β-D- Glucopyranosyl platycogenate A C39H62O13 C CH3 Glc CH CH3 [16] 69 Dimethyl 3-O-β-D-glucopyranosyl Platycogenate A C38H60O13 C CH3 Glc H CH3 [16] 70 Platycoside Q C53H82O25 D GEN S2 - - [14] 71 Platycoside M-1 C36H54O12 D Glc H - - [21] 72 Platycoside M-2 C47H72O20 D Glc S2 - - [21] 73 Platycoside M-3 C52H80O24 D Glc S3 - - [21] 74 Platyconic acid A lactone C57H88O29 D Glc S4 - - [5] 75 Platyconic acid B lactone C63H98O34 D GEN S4 - - [9] 76 Deapi-platyconic acid A lactone C52H80O25 D Glc S3 - - [5] 77 Deapi-platyconic acid B lactone C58H90O30 D GEN S3 - - [9] 78 Platycogenic acid A lactone C30H44O8 D H H - - [5] 79 3-O-β-D-glucopyranosyl platycogenic acid A lactone methyl ester C37H56O12 D Glc CH3 - - [16] 80 Platycodonoids A C29H46O5 E H H - - [15] 81 Platycodonoids B C35H56O10 E Glc H - - [15] 82 16-Oxo-platycodin D C57H90O28 E Glc S4 - - [22] 83 Platycodsaponin A C42H68O16 F CH3 Glc Glc - [6] 84 Platycogenic acid B C30H46O8 F COOH H H - [3] 85 Platycogenic acid C C30H48O6 F CH3 H H - [3] 86 3-O-β-D-glucopyranosyl-2β, 12α, 16α, 23, 24-pentahydroxy-oleanane-28(13)-lactone C36H58O13 G CH2OH Glc - - [23] 87 3-O-β-D-glucopyranosyl-(1→3)-β-D-glucopyranosyl-2β, 12α, 16α, 23α-tetrahydroxy-oleanane-28(13)-lactone C42H68O17 G CH3 LAM - - [23] 88 Platycodon A C42H68O16 H Glc - - - [24] 89 Platycodon B C41H66O15 H Xyl - - - [24] 注:S1=Glc6-Glc6-Glc; S2=Ara2-rha;S3=Ara2-rha4-xyl;S4=Ara2-rha4-xyl3-api;S5=Ara2-rha(3-OAc)4-xyl;S6=Ara2-rha(2-OAc)4-xyl;S7=Ara2-rha(3-OAc)4-xyl3-api;S8=Ara2-rha(2-OAc)4-xyl3-api;S9=Ara2-rha(2-OAc);Glc=β-D-glucopyranosyl;Ara=α-L-arabinopyranosyl;Rha=α-L-rhamnopyranosyl;Xyl=β-D-xylopyranosyl;Api=β-D-apiofuranosyl;GlcA= D-glucuronic acid LAM(Laminaribiose)=Glc3-Glc;GEN(gentiobiose)=Glc6-Glc。 -
[1] 国家药典委员会. 中华人民共和国药典: 一部[S]. 北京: 中国医药科技出版社, 2020. [2] 张岩, 魏建和, 刘娟, 等. 三大主产地桔梗营养成分分析及评价[J]. 中国现代中药, 2019, 21(2), 21: 194-198. https://www.cnki.com.cn/Article/CJFDTOTAL-YJXX201902011.htm [3] KUBOTA T, KITATANI H, HINOH H. The structure of platycogenic acids A, B, and C, further triterpenoid constituents of Platycodon grandiflorum A. De Candolle[J]. J Chem Soc D, 1969(22): 1313. doi: 10.1039/c29690001313 [4] HA YW, NA YC, HA IJ, et al. Liquid chromatography/mass spectrometry-based structural analysis of new platycoside metabolites transformed by human intestinal bacteria[J]. J Pharm Biomed Anal, 2010, 51(1): 202-209. doi: 10.1016/j.jpba.2009.08.002 [5] CHOI YH, YOO DS, CHOI CW, et al. Platyconic acid A, a genuine triterpenoid saponin from the roots of Platycodon grandiflorum[J]. Molecules, 2008, 13(11): 2871-2879. doi: 10.3390/molecules13112871 [6] IDA Y, FUKUMURA M, IWASAKI D, et al. Eight new oleanane-type triterpenoid saponins from Platycodon root[J]. Heterocycles, 2010, 81(12): 2793. doi: 10.3987/COM-10-12058 [7] NA YC, HA YW, KIM YS, et al. Structural analysis of platycosides in Platycodi Radix by liquid chromatography/electrospray ionization-tandem mass spectrometry[J]. J Chromatogr A, 2008, 1189(1/2): 467-475. http://www.onacademic.com/detail/journal_1000034061541810_c2ab.html [8] JEONG EK, HA IJ, KIM YS, et al. Glycosylated platycosides: Identification by enzymatic hydrolysis and structural determination by LC-MS/MS[J]. J Sep Sci, 2014, 37(1/2): 61-68. http://www.onacademic.com/detail/journal_1000036930131710_305e.html [9] CHOI YH, YOO DS, CHA MR, et al. Antiproliferative effects of saponins from the roots of Platycodon grandiflorum on cultured human tumor cells[J]. J Nat Prod, 2010, 73(11): 1863-1867. doi: 10.1021/np100496p [10] NIKAIDO T, KOIKE K, MITSUNAGA K, et al. Triterpenoid saponins from root of Platycodon grandiflorum[J]. Nat Med, 1998, 52: 54-9. [11] NIKAIDO T, KOIKE K, MITSUNAGA K, et al. Two new triterpenoid saponins from Platycodon grandiflorum[J]. Chem Pharm Bull, 1999, 47(6): 903-904. doi: 10.1248/cpb.47.903 [12] FU WW, SHIMIZU N, DOU DQ, et al. Five new triterpenoid saponins from the roots of Platycodon grandiflorum[J]. Chem Pharm Bull (Tokyo), 2006, 54(4): 557-560. doi: 10.1248/cpb.54.557 [13] HE ZD, QIAO CF, HAN QB, et al. New triterpenoid saponins from the roots of Platycodon grandiflorum[J]. Tetrahedron, 2005, 61(8): 2211-2215. doi: 10.1016/j.tet.2004.12.032 [14] QIU L, XIAO Y, LIU YQ, et al. Platycosides P and Q, two new triterpene saponins from Platycodon grandiflorum[J]. J Asian Nat Prod Res, 2019, 21(5): 419-425. doi: 10.1080/10286020.2018.1488835 [15] ZHAN Q, ZHANG F, SUN LN, et al. Two new oleanane-type triterpenoids from Platycodi Radix and anti-proliferative activity in HSC-T6 cells[J]. Molecules, 2012, 17(12): 14899-14907. doi: 10.3390/molecules171214899 [16] ISHⅡ H, TORI K, TOZYO T, et al. Saponins from roots of Platycodon grandiflorum. Part 1. Structure of prosapogenins[J]. J Chem Soc, 1981, 1981: 1928. doi: 10.1002/chin.198143295/abstract [17] LI W, ZHANG W, XIANG L, et al. Platycoside N: A new oleanane-type triterpenoid saponin from the roots of Platycodon grandiflorum[J]. Mol Basel Switz, 2010, 15(12): 8702-8708. http://www.onacademic.com/detail/journal_1000040539747910_56b7.html [18] 付文卫, 侯文彬, 窦德强, 等. 桔梗中远志酸型皂苷的化学研究[J]. 药学学报, 2006, 41(4): 358-360. doi: 10.3321/j.issn:0513-4870.2006.04.013 [19] FU WW, FU JN, ZHANG WM, et al. Platycoside O, a new triterpenoid saponin from the roots of Platycodon grandiflorum[J]. Molecules, 2011, 16(6): 4371-4378. doi: 10.3390/molecules16064371 [20] ISHⅡ H, TORI K, TOZYO T, et al. Saponins from roots of Platycodon grandiflorum. Part 2. Isolation and structure of new triterpene glycosides[J]. J Chem Soc, 1984(10): 661-668. doi: 10.1002/chin.198429313/abstract [21] FU WW, SHIMIZU N, TAKEDA T, et al. New A-ring lactone triterpenoid saponins from the roots of Platycodon grandiflorum[J]. Chem Pharm Bull (Tokyo), 2006, 54(9): 1285-1287. doi: 10.1248/cpb.54.1285 [22] LI W, XIANG L, ZHANG J, et al. A new triterpenoid saponin from the roots of Platycodon grandiflorum[J]. Chin Chem Lett, 2007, 18(3): 306-308. doi: 10.1016/j.cclet.2007.01.009 [23] ZHANG L, LIU ZH, TIAN JK. Cytotoxic triterpenoid saponins from the roots of Platycodon grandiflorum[J]. Molecules, 2007, 12(4): 832-841. doi: 10.3390/12040832 [24] MA GX, GUO WJ, ZHAO LZ, et al. Two new triterpenoid saponins from the root of Platycodon grandiflorum[J]. Chem Pharm Bull (Tokyo), 2013, 61(1): 101-104. doi: 10.1248/cpb.c12-00713 [25] GOTO T, TADAOKONDO, TAMURA H, et al. Structure of platyconin, a diacylated anthocyanin isolated from the Chinese bell-flower Platycodon grandiflorum[J]. Tetrahedron Lett, 1983, 24(21): 2181-2184. doi: 10.1016/S0040-4039(00)81877-8 [26] INADA A, MURATA H, SOMEKAWA M, et al. Phytochemical studies of seeds of medicinal plants Ⅱ: A new dihydroflavonol glycoside and a new 3-methyl-1-butanol glycoside from seeds of Platycodon grandiflorum A. DE CANDOLLE[J]. Chem Pharm Bull, 1992, 40(11): 3081-3083. doi: 10.1248/cpb.40.3081 [27] 李凌军, 刘振华, 陈赟, 等. 桔梗的化学成分研究[J]. 中国中药杂志, 2006(18): 1506-1509. doi: 10.3321/j.issn:1001-5302.2006.18.007 [28] LEE JY, YOON JW, KIM CT, et al. Antioxidant activity of phenylpropanoid esters isolated and identified from Platycodon grandiflorum A. DC[J]. Phytochemistry, 2004, 65(22): 3033-3039. doi: 10.1016/j.phytochem.2004.08.030 [29] MAZOL I, GLESK M, CISOWSKI W. Polyphenolic compounds from Platycodon grandiflorum A. DC[J]. Acta Pol Pharm, 2004, 61(3): 203-208. http://ptf.content-manager.pl/pub/File/wydawnictwa/acta_pol_2004/pdf-y%202004-3/203-208.pdf [30] OKA M, OTA N, MINO Y, et al. Studies on the conformational aspects of inulin oligomers[J]. Chem Pharm Bull (Tokyo), 1992, 40(5): 1203-1207. doi: 10.1248/cpb.40.1203 [31] 宫勋, 王建刚. 桔梗中脂肪酸成分的GC-MS分析[J]. 安徽农业科学, 2010, 38(22): 11780-11782. doi: 10.3969/j.issn.0517-6611.2010.22.045 [32] CHEN B, LIU ZB, ZHANG YW, et al. Application of high-speed counter-current chromatography and HPLC to separate and purify of three polyacetylenes from Platycodon grandiflorum[J]. J Sep Sci, 2018, 41(3): 789-796. doi: 10.1002/jssc.201700767 [33] SHIN CY, LEE WJ, LEE EB. et al. Platycodin D and D3 increase airway mucin release in vivo and in vitro in rats and hamsters[J]. Planta Med, 2002, 68: 221-225. doi: 10.1055/s-2002-23130 [34] RYU J, LEE HJ, PARK SH, et al. Effects of the root of Platycodon grandiflorum on airway mucin hypersecretion in vivo and platycodin D(3) and deapi-platycodin on production and secretion of airway mucin in vitro[J]. Phytomedicine, 2014, 21(4): 529-533. doi: 10.1016/j.phymed.2013.10.004 [35] GAO W, GUO Y, YANG HX. Platycodin D protects against cigarette smoke-induced lung inflammation in mice[J]. Int Immunopharmacol, 2017, 47: 53-58. doi: 10.1016/j.intimp.2017.03.009 [36] FU YH, XIN ZY, LIU B, et al. Platycodin D inhibits inflammatory response in LPS-stimulated primary rat microglia cells through activating LXRα-ABCA1 signaling pathway[J]. Front Immunol, 2017, 8: 1929. http://europepmc.org/articles/PMC5767310/ [37] ZHANG ZY, ZHAO MC, ZHENG WX, et al. Platycodin D, a triterpenoid saponin from Platycodon grandiflorum, suppresses the growth and invasion of human oral squamous cell carcinoma cells via the NF-κB pathway[J]. J Biochem Mol Toxicol, 2017, 31(9): 1-10. http://europepmc.org/abstract/MED/28548219 [38] LI Y, WU YY, XIA Q, et al. Platycodon grandiflorus enhances the effect of DDP against lung cancer by down regulating PI3K/Akt signaling pathway[J]. Biomed Pharmacother, 2019, 120: 109496. doi: 10.1016/j.biopha.2019.109496 [39] HWANG KA, HWANG YJ, IM PR, et al. Platycodon grandiflorum extract reduces high-fat diet-induced obesity through regulation of adipogenesis and lipogenesis pathways in mice[J]. J Med Food, 2019, 22(10): 993-999. doi: 10.1089/jmf.2018.4370 [40] LEE EJ, KANG M, KIM YS. Platycodin D inhibits lipogenesis through AMPKα-PPARγ2 in 3T3-L1 cells and modulates fat accumulation in obese mice[J]. Planta Med, 2012, 78(14): 1536-1542. doi: 10.1055/s-0032-1315147 [41] KHANAL T, CHOI JH, HWANG YP, et al. Protective effects of saponins from the root of Platycodon grandiflorum against fatty liver in chronic ethanol feeding via the activation of AMP-dependent protein kinase[J]. Food Chem Toxicol, 2009, 47(11): 2749-2754. doi: 10.1016/j.fct.2009.08.006 [42] KE WX, WANG P, WANG XH, et al. Characterization of inulin-type fructan from platycodon grandiflorus and study on its prebiotic and immunomodulating activity[J]. Nutrients, 2020, 12(2): E480. doi: 10.3390/nu12020480 [43] LIU YM, CONG S, CHENG Z, et al. Platycodin D alleviates liver fibrosis and activation of hepatic stellate cells by regulating JNK/c-JUN signal pathway[J]. Eur J Pharmacol, 2020, 876: 172946. doi: 10.1016/j.ejphar.2020.172946 [44] SHENG Y, LIU G, WANG M, et al. A selenium polysaccharide from Platycodon grandiflorum rescues PC12 cell death caused by H2O2 via inhibiting oxidative stress[J]. Int J Biol Macromol, 2017, 104(pt a): 393-399. http://www.onacademic.com/detail/journal_1000039929369610_9221.html [45] SHI CY, LI Q, ZHANG XY. Platycodin D protects human fibroblast cells from premature senescence induced by H2O2 through improving mitochondrial biogenesis[J]. Pharmacology, 2020, 105(9/10): 598-608. http://www.researchgate.net/publication/338961900_Platycodin_D_Protects_Human_Fibroblast_Cells_from_Premature_Senescence_Induced_by_H2O2_through_Improving_Mitochondrial_Biogenesis [46] PANG DJ, HUANG C, CHEN ML, et al. Characterization of inulin-type fructan from Platycodon grandiflorus and study on its prebiotic and immunomodulating activity[J]. Molecules, 2019, 24(7): 1199. doi: 10.3390/molecules24071199 [47] NOH EM, KIM JM, LEE HY, et al. Immuno-enhancement effects of Platycodon grandiflorum extracts in splenocytes and a cyclophosphamide-induced immunosuppressed rat model[J]. BMC Complement Altern Med, 2019, 19(1): 322. doi: 10.1186/s12906-019-2724-0 [48] LUO Q, WEI GY, WU XQ, et al. Platycodin D inhibits platelet function and Thrombus formation through inducing internalization of platelet glycoprotein receptors[J]. J Transl Med, 2018, 16(1): 311. doi: 10.1186/s12967-018-1688-z [49] CHOI YJ, LEE SJ, KIM HI, et al. Platycodin D enhances LDLR expression and LDL uptake via down-regulation of IDOL mRNA in hepatic cells[J]. Sci Rep, 2020, 10(1): 19834. doi: 10.1038/s41598-020-76224-w [50] ZHANG WZ, HOU JG, YAN XT, et al. Platycodon grandiflorum saponins ameliorate cisplatin-induced acute nephrotoxicity through the NF-κB-mediated inflammation and PI3K/Akt/apoptosis signaling pathways[J]. Nutrients, 2018, 10(9): E1328. doi: 10.3390/nu10091328 [51] KIM JI, JEON SG, KIM KA, et al. Platycodon grandiflorus root extract improves learning and memory by enhancing synaptogenesis in mice Hippocampus[J]. Nutrients, 2017, 9(7): E794. doi: 10.3390/nu9070794 [52] KIM YA, JIN SW, OH SH, et al. Platycodon grandiflorum-derived saponin enhances exercise function, skeletal muscle protein synthesis, and mitochondrial function[J]. Food Chem Toxicol, 2018, 118: 94-104. doi: 10.1016/j.fct.2018.04.062 [53] SHAN JJ, ZOU JS, XIE T, et al. Pharmacokinetics, intestinal absorption and microbial metabolism of single platycodin D in comparison to Platycodi Radix extract[J]. Pharmacogn Mag, 2015, 11(44): 750-755. doi: 10.4103/0973-1296.165576 [54] SHAN JJ, ZOU JS, XIE T, et al. Effects of Gancao on pharmacokinetic profiles of platycodin D and deapio-platycodin D in Jiegeng[J]. J Ethnopharmacol, 2015, 170: 50-56. doi: 10.1016/j.jep.2015.04.056 [55] KWON M, JI HK, GOO SH, et al. Involvement of intestinal efflux and metabolic instability in the pharmacokinetics of platycodin D in rats[J]. Drug Metab Pharmacokinet, 2017, 32(5): 248-254. doi: 10.1016/j.dmpk.2017.05.005 [56] CHA SB, LI Y, BAE JS, et al. Evaluation of 13-week subchronic toxicity of Platycodon grandiflorus (Jacq. ) A. DC. root extract in rats[J]. J Ethnopharmacol, 2021, 267: 113621. doi: 10.1016/j.jep.2020.113621 [57] LEE WH, GAM CO, KU SK, et al. Single oral dose toxicity test of platycodin d, a saponin from platycodin Radix in mice[J]. Toxicol Res, 2011, 27(4): 217-224. doi: 10.5487/TR.2011.27.4.217 [58] SUN HX, CHEN LQ, WANG JJ, et al. Structure-function relationship of the saponins from the roots of Platycodon grandiflorum for hemolytic and adjuvant activity[J]. Int Immunopharmacol, 2011, 11(12): 2047-2056. doi: 10.1016/j.intimp.2011.08.018 [59] 谭玲玲, 侯晓敏, 胡正海. 不同产地桔梗药材中桔梗总皂苷和桔梗皂苷D的测定[J]. 中草药, 2015, 46(11): 1682-1684. https://www.cnki.com.cn/Article/CJFDTOTAL-ZCYO201511023.htm [60] 蒋龄周, 龚祖芳. 一测多评法同时测定桔梗中3种桔梗皂苷的含量[J]. 中国现代应用药学, 2017, 34(5): 729-732. https://www.cnki.com.cn/Article/CJFDTOTAL-XDYD201705023.htm [61] 喻格, 朱丽丽, 张玲. 气相色谱-质谱联用法测定桔梗中挥发油成分[J]. 中药新药与临床药理, 2020, 31(11): 1373-1378. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYXY202011017.htm [62] 司雨柔, 高韵, 解玫莹, 等. 不同产地桔梗的红外光谱整体成分鉴别研究[J]. 化学试剂, 2021, 43(2): 210-215. https://www.cnki.com.cn/Article/CJFDTOTAL-HXSJ202102015.htm [63] 陈宝, 王燕华, 王玉方, 等. UPLC法同时测定不同产地桔梗中13种核苷类成分[J]. 中药材, 2018, 41(2): 381-384. https://www.cnki.com.cn/Article/CJFDTOTAL-ZYCA201802030.htm [64] 许传莲, 郑毅男, 杨腊虎, 等. HPLC法测定不同采收期及不同部位桔梗中桔梗皂苷D含量[J]. 吉林农业大学学报, 2001, 23(1): 58-60, 64. doi: 10.3969/j.issn.1000-5684.2001.01.016 [65] 宋健, 包华音, 王颖, 等. 桔梗生长年限和采收期与质量的相关性研究[J]. 齐鲁药事, 2011, 30(6): 313-315. https://www.cnki.com.cn/Article/CJFDTOTAL-SDYG201106001.htm [66] 黄力, 金传山, 吴德玲. 不同干燥方法对桔梗中桔梗皂苷D含量的影响[J]. 安徽中医学院学报, 2010, 29(3): 69-71. doi: 10.3969/j.issn.1000-2219.2010.03.025 [67] 付志文, 王玲, 董其亭, 等. 不同加工工艺对桔梗浸出物及总皂苷含量的影响[J]. 中国中药杂志, 2008, 33(5): 579-580. doi: 10.3321/j.issn:1001-5302.2008.05.026 [68] 曾静凯, 郭青. 不同产地桔梗性状、浸出物、桔梗皂苷D含量及HPLC指纹图谱比较[J]. 中国实验方剂学杂志, 2017, 23(24): 62-70. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX201724011.htm [69] 张铁军, 白钢, 刘昌孝. 中药质量标志物的概念、核心理论与研究方法[J]. 药学学报, 2019, 54(2): 187-196, 186. https://www.cnki.com.cn/Article/CJFDTOTAL-YXXB201902002.htm [70] 单进军, 邹葭霜, 徐建亚, 等. 桔梗汤的研究进展[J]. 中国实验方剂学杂志, 2012, 18(19): 304-306. https://www.cnki.com.cn/Article/CJFDTOTAL-ZSFX201219087.htm -





 下载:
下载: